Leaderboard
-
Points8,959Posts
-
 Points279Posts
Points279Posts -
Points1,581Posts
-

Popular Content
Showing content with the highest reputation since 06/29/2021 in all areas
-
Storing Saline Cubes
Storing Saline Cubes
jshepherd and 16 others reacted to John C. Staley for a post in a topic
17 pointsI'm going to be blunt. This is ridiculous!! You have the potential of causing far more problems by removing the cubes from their protective container.17 points -
Plasma Freezer Down: Better Process for Temp Storage of Frozen Products
Plasma Freezer Down: Better Process for Temp Storage of Frozen Products
wellspl and 11 others reacted to jayinsat for a post in a topic
12 pointsAll, I am about to blow your mind.... Our plasma freezer is down and so is our backup. The freezer will not get colder than -18 C. I was preparing to move all the products into boxes with dry ice until I had a conversation with my 87 year old dad, a retired blood banker from University of Chicago. He said to me, do not take the plasma out of the freezer and put it in boxes, PUT THE DRY ICE IN THE FREEZER, IT IS THE BEST STORAGE BOX YOU HAVE!!!! MIND=BLOWN!!!! I did that. Our freezer is currently reading -25.1C and getting colder. Furthermore, the probes in the freezer continually monitor the temp in the freezer so you don't have to record temps every 4 hours, the chart is doing that for you!!! Isn't that cool? That perfectly illustrates the difference between wisdom and knowledge there. I wish we could hire my dad. I just had to share this here. PS. Freezer is now at -26.4C.12 points -
+s in Ortho panel
+s in Ortho panel
traci89 and 10 others reacted to Malcolm Needs for a post in a topic
11 pointsThe +s stands for strongly expressed. The expression of the P1 antigen varies considerably from person to person, but the reaction strength with anti-P1 is an inherited trait (i.e. the strength of the expression on the red cell surface). "I apologize for this dumb question." BBnoob69, NO QUESTION IS A DUMB QUESTION, IF YOU DO NOT KNOW THE ANSWER. If you don't know the answer, the dumb thing is to not ask the question in the first place. NEVER be afraid to ask a question on here,11 points -
Incompatible Blood
Incompatible Blood
tesSBB and 10 others reacted to jayinsat for a post in a topic
11 pointsYou did everything that was required in this situation. The patient was a trauma and needed emergency transfusion. The risk of death outweighed the risk of a hemolytic transfusion reaction in that scenario, according to the treating physician. I once had a trauma surgeon tell me "I can treat a transfusion reaction but I can't treat death!" That put things in perspective for me. That is why thy sign the consent. Next step would be to report this to your risk management department so that follow-up can be made, including monitoring the patient for the s/s of DTR.11 points -
Mock-up cases
Mock-up cases
ElinF and 9 others reacted to Bb_in_the_rain for a post in a topic
10 pointsFor those of who works in transfusion service laboratory and would like to learn more reference cases, I can post some mock-up cases here. If you would like me to do it, please hit the "heart" button on this post. If enough folks want to practice case studies on reference lab cases, I can post mock-up cases here weekly or so..10 points -
Do you antigen type for the entire group?
Do you antigen type for the entire group?
SbbPerson and 9 others reacted to Malcolm Needs for a post in a topic
10 pointsWhen I was working in the Reference Laboratory at the NHSBT and, come to that, when I was working for a short time in a Hospital Blood Bank, we would ALWAYS test for the C, c, E and e antigens, together with the K antigen, both for patients and donors, and we would also test for the antithetical antigen, as well as the cognate antigen (in other words, as in your example, the Jk(a) and the Jk(b) antigen. We ALWAYS did this, except when the grouping reagent was exceedingly rare (e.g. anti-Dib) or the antibody AND the antigen were extremely rare (e.g. anti-Kpc). The reason we did this, particularly in the NHSBT Reference Laboratory, was because we wanted to identify very rare phenotypes, such as Kp(a+b-), or even rarer (in most cases), null phenotypes, but there was also a paper that showed that people who were transfusion dependent, such as sicklers and thal patients tend, once they have made an initial atypical antibody (particularly anti-C, anti-c, anti-E, anti-e or anti-K) to make all sorts of specificities (I'll try to look up the paper and get back to you on here). Other papers comparing their findings actually agreed with them. I say ALWAYS, but then, of course, the Bean Counters, who know nothing about Blood Group Serology, or about Patient Requirements, and care even less, came along, and we were banned from doing this as, apparently, IT COST TOO MUCH MONEY, except in special circumstances, such as patients from the Black populations, where we were privileged to be able to test for both Fya AND Fyb, in case they were Fy(a-b-) - and, of course, most of those who were found to be Fy(a-b-) had the FYB gene, so would very rarely produce an anti-Fy3, as they were homozygous for the GATA1 gene mutation. Unfortunately, what these "suits" seem to forget, despite counting beans for a living, is that, if the patient goes on to produce other, clinically significant, atypical alloantibodies, they will occupy a hospital bed for longer while suitable blood is identified, including, sometimes, cryopreserved units, ALL OF WHICH IS FAR MORE EXPENSIVE THAN THE INITIAL TYPING WAS IN THE FIRST PLACE - but what do we professionals know! RANT OVER!!!!!!!!!!!!!!!!10 points -
Immune checkpoint inhibitor drugs can cause DAT negative AIHA
Immune checkpoint inhibitor drugs can cause DAT negative AIHA
donellda and 9 others reacted to Mabel Adams for a post in a topic
10 pointsWe had a melanoma patient on Nivolumab = Opdivo who apparently has hemolytic anemia but his IgG was only microscopically positive and his complement was negative. Hgb 5.5. Retic % slightly elevated, absolute retic normal, immature fraction retic very high. Bili and LDH normal. Hpt <14 and responded to steroids. They blamed this drug so I hunted up this article. This was new to me so I wanted to share it. Clinical Trial Am J Hematol 2019 May;94(5):563-574. doi: 10.1002/ajh.25448. Epub 2019 Mar 13. Clinical and laboratory features of autoimmune hemolytic anemia associated with immune checkpoint inhibitors Rebecca Karp Leaf 1, Christopher Ferreri 2, Deepa Rangachari 3, James Mier 3, Wesley Witteles 4, George Ansstas 5, Theodora Anagnostou 6, Leyre Zubiri 1, Zofia Piotrowska 1, Thein H Oo 7, David Iberri 8, Mark Yarchoan 9, April K S Salama 10, Douglas B Johnson 11, Andrew D Leavitt 12, Osama E Rahma 13, Kerry L Reynolds 1, David E Leaf 14 PMID: 30790338 DOI: 10.1002/ajh.25448 Free article Abstract Immune checkpoint inhibitors (ICPis) are a novel class of immunotherapeutic agents that have revolutionized the treatment of cancer; however, these drugs can also cause a unique spectrum of autoimmune toxicity. Autoimmune hemolytic anemia (AIHA) is a rare, but often severe, complication of ICPis. We identified 14 patients from nine institutions across the United States who developed ICPi-AIHA. The median interval from ICPi initiation to development of AIHA was 55 days (interquartile range [IQR], 22-110 days). Results from the direct antiglobulin test (DAT) were available for 13 of 14 patients: 8 patients (62%) had a positive DAT and 5 (38%) had a negative DAT. The median pretreatment and nadir hemoglobin concentrations were 11.8 g/dL (IQR, 10.2-12.9 g/dL) and 6.3 g/dL (IQR, 6.1-8.0 g/dL), respectively. Four patients (29%) had a preexisting lymphoproliferative disorder, and two (14%) had a positive DAT prior to initiation of ICPi therapy. All patients were treated with glucocorticoids, with three requiring additional immunosuppressive therapy. Complete and partial recoveries of hemoglobin were achieved in 12 (86%) and 2 (14%) patients, respectively. Seven patients (50%) were re-challenged with ICPis, and one (14%) developed recurrent AIHA. Clinical and laboratory features of ICPi-AIHA were similar in DAT positive and negative patients. ICPi-AIHA shares many clinical features with primary AIHA; however, a unique aspect of ICPi-AIHA is a high incidence of DAT negativity. Glucocorticoids are an effective first-line treatment in the majority of patients with ICPi-AIHA, and most patients who are re-challenged with an ICPi do not appear to develop recurrence of AIHA.10 points -
Storing Saline Cubes
Storing Saline Cubes
jshepherd and 9 others reacted to albaugh for a post in a topic
10 pointsI think you should invite members of that committee to remove a bag from its cube, try to label it sufficiently (substance, lot #, expiration, etc.), attach that label in such a way that it will stay attached when the bag 'collapses' as it's emptied, hoist the bag up to the level of a cell washer without the aid of the box (especially this part), and suggest ways to keep the collapsed bag at an angle that will ensure all the contents are used. I'm willing to bet they'll come around.10 points -
Serological Crossmatch when providing Antigen Negative units
Serological Crossmatch when providing Antigen Negative units
AuntiS and 9 others reacted to Malcolm Needs for a post in a topic
10 pointsNo, there is a lot more to it than that. Anti-A and anti-B are isoantibodies, rather than alloantibodies. In other words, they are "naturally occurring" and do not have to be stimulated by either red cell transfusion or pregnancy. They are usually stimulated by particles in the air (including human cells that have been shed into the air) that either express chemical compounds that mimic the A and/or B antigens or, in the case of shed human cells, actually do express these antigens (remember, the A, B and H antigens are histoantigens). On the other hand, genuine alloantibodies (for example, let's say an anti-Jka) that are stimulated by transfusions and/or pregnancy, have, by definition, shown the individual to be a "responder". It is by no means unusual for an individual who has produced a genuine alloantibody (such as the anti-Jka mentioned above) to produce an alloantibody of another specificity (or alloantibodies of other specificities). Such other alloantibodies may not be easily detectable by routine serological techniques for various reasons. Three of these are that the antibodies may not become serologically detectable at the same time (one may be detectable as early as the other, as not all antibodies "read the books"), that an antibody may be evanescent (or "disappears" from the circulation quite quickly - such as many Kidd antibodies - but these can remain clinically significant if re-stimulated), and thirdly, that the cognate antigen is not expressed on either the screening red cells or the red cells used in the antibody identification panel (for example, in the UK, to give two examples, the Jsa antigen and the Wra antigen, and both of these antibodies can be exceedingly clinically significant). For this reason, it is very important that a serological cross-match is preformed (and found to be compatible), even if the blood provided is antigen negative for a known cognate antibody. You may well ask, "Well, what about an anti-Wra (for example) that is present as a monospecific antibody? Is that not clinically significant?", and the answer is "Yes"! Indeed, there was a fatal case of an acute transfusion reaction caused by anti-Wra within the last decade in the UK, and the court decided that it was death by misadventure, because anti-Wra is known to be quite a common antibody, whereas the cognate antigen is sufficiently rare for the Law to recognise that it does not need to be expressed on screening cells (otherwise the Reference Laboratories would be overwhelmed with samples that have anti-Wra in their plasma/serum - and this is only one such specificity). This may be one of the few times that our judiciary have used their brains (did I say that??????????!!!!!!!!!!!!!!!!!) and the decision may have been influenced by an editorial in Transfusion, written by the late, great Professor George Garratty (Garratty G. How concerned should we be about missing antibodies to low incidence antigens? Transfusion 2003; 43(7): 844-847. DOI: 10.1046/j.1537-2995.2003.00492.x.). SORRY THIS IS A BIT (VERY) LENGTHY!10 points -
Use of plastic tubes for tube testing
Use of plastic tubes for tube testing
AuntiS and 9 others reacted to exlimey for a post in a topic
10 pointsThis issue - the switch to plastic - seems to bubble up every few years (pardon the minor pun). When I was a puppy in my early years, last century, labs were already tossing around the idea to avoid potentially dangerous, sharp glass tubes. When broken, the plastic used for test tubes is also sharp, possibly worse that glass, as Malcolm suggests. As others have mentioned, static is always an issue with the plastic version, rather than occasional with glass. Other than that, and in my experience, plastic test tubes tubes work almost as well as glass for serological testing. However, many "tube reagents" are not formulated for, or qualified in plastic. The Directions for Use/ Package Inserts may be restrictive. Two points - personal opinion of a cranky old man: 1. One event does not indicate a trend - changing the whole system to address a single cut-finger incident is unreasonable. 2. The various safety apparatuses (however they be mis- or confusingly named) exist to limit institutional legal liability, i.e., prevention of legal action ("please don't sue us"). The workers' actual safety is often secondary.10 points -
Use of plastic tubes for tube testing
Use of plastic tubes for tube testing
jojo808 and 9 others reacted to Ensis01 for a post in a topic
10 pointsAh, inform them that by their logic; phlebotomist's should not use needles due to the many unintended sticks in hospitals each year10 points -
Transfusion requirement for patient of A3 subgroup?
Transfusion requirement for patient of A3 subgroup?
BB Gal and 8 others reacted to Malcolm Needs for a post in a topic
9 pointsThere is absolutely no reason to give group O red cells to a recipient who is A3. Even if the patient does develop an anti-A1, unless that antibody is reactive at strictly 37oC, they can still receive A1 red cells, but, if the anti-A1 does react at 37oC, there is no reason not to transfuse with A2 red cells that are IAT compatible. Personally, I have never seen loss of A or B antigens through ALL, but I have with AML. In fact, in one case, we were able to follow whether the patient was in remission or relapse by the strength of the reaction of the A antigen with various anti-A reagents, but this was many years ago, and I honestly can't remember whether these were human-derived polyclonal reagents or early monoclonal reagents.9 points -
CPDA-1 Blood
CPDA-1 Blood
Judes and 8 others reacted to Neil Blumberg for a post in a topic
9 pointsOur Red Cross just informed us that it will discontinue providing CPDA-1 rbc. We primarily used it to provide volume reduced red cells to pediatric patients under 3 years of age. We will volume reduce AS-1 or AS-3 by centrifugation or washing (Terumo 2991) instead. Probably unnecessary for most patients, but this is a long standing practice here, and it doesn't seem worthwhile trying to adjust pediatric practice in this regard. Most patients do not need the additional volume provided by the anticoagulant-preservative in AS-1, etc., and avoiding unnecessary volume is a reasonable goal in many patients. There is no inherent virtue to CPDA-1 vs. AS-1 and similar solutions, and rbc preservation is slightly better in AS-1/AS-3 by in vitro metrics. There is absolutely no factual basis for using CPD-A1 in preference to AS-1, etc. in pediatrics. Purely expert opinion and probably unduly conservative. I've attached a nice presentation by Dr. Saifee at the University of Washington, who createdAdditive solution AS-1 in Children Univ. Washington presentation Dec 2021.pptx it to educate her colleagues about using AS-1 instead of CPDA-1. Additive solution AS-1 in Children Univ. Washington presentation Dec 2021.pptx Pediatric RBC White Paper - November 2021.pdf9 points -
Confirm anti-D (vs anti-G) via titers?
Confirm anti-D (vs anti-G) via titers?
SbbPerson and 8 others reacted to Malcolm Needs for a post in a topic
9 pointsIt is usual for the C+, D- red cells (e.g. r'r) to react with an anti-G more strongly than a C-, D+ red cell (e.g. R2R2), BUT, this is by no means "diagnostic". As Jsbneg says above, it would be far safer to perform the proper tests, to ensure you have ascertained the correct specificity/specificities. The attached PowerPoint may or may not help (ignore if it is not helpful). The G Antigen and Anti G.pptx9 points -
MTP cut-off policy, or Lethal Dose of Blood Products
MTP cut-off policy, or Lethal Dose of Blood Products
Mabel Adams and 8 others reacted to Neil Blumberg for a post in a topic
9 pointsThere are no data suggesting a particular limit. Survival is very unusual after 30-50 units of red cells, but everyone has exceptional cases like those mentioned above. We have discussed futility of care many times, and our practitioners are quite amenable and forthcoming. We have stopped resuscitation in a young man having a liver transplant go badly, when there was no surgical path to hemostasis after about 250 units, but this is unusual too. Bottom line, a case by case decision as to whether care is futile and/or the patient's needs endanger the well being of other patients needing transfusion. Those are the key issues in each case to my way of thinking.9 points -
MTP cut-off policy, or Lethal Dose of Blood Products
I have issued 148 units of products to a guy who was cycle vs car massive haemorrhage - he survived. I have issues 120ish units on an obstetric massive haemorrhage (as well as 20 6-packs on the twins) - all 3 survived. I've issued similar on AAA (with eventual bypass) - survival. I think the key is to use TEG to see whether the clotting is screwed - if they are clotting then keep going... In the grand scheme of things blood is cheap9 points
-
Emergency Neonatal Transfusion in Small Hospitals
Emergency Neonatal Transfusion in Small Hospitals
saralm88 and 8 others reacted to Neil Blumberg for a post in a topic
9 pointsThere is reason NOT to use the freshest possible units. They may be more toxic than intermediate stored units. This is something that made sense but was almost certainly wrong. See below for the reasoning and published data. We use <21 days as fresh for this reason and avoid <7 days storage for everyone based upon the randomized trial data. BMJ 2019;366:l4968 doi: 10.1136/bmj.l4968 (Published 5 August 2019) Page 1 of 1 Letters Trivella and colleagues present some caveats around the subject of duration of red cell storage and clinical outcomes.1 Studies have been widely interpreted as showing that transfusion is not associated with adverse clinical outcomes. I think this is a serious misinterpretation of the data. In addition to the concerns raised by the authors, another valid hypothesis, which has received little attention, is that very short storage red cells might be more dangerous than medium storage periods (say 7-21 days) and equally dangerous as longer storage red cells (say 28-42 days). An inverted U shaped curve. The evidence for this comes from a meta-analysis finding that “ultra short” storage of red cells was associated with a post-transfusion increase in nosocomial infection.2 Shorter storage red cells have a greater imbalance of oxidation-reduction potential than longer storage red cells in preliminary studies in vitro.3 Red cell storage duration is also a poor predictor of post-transfusion free haemoglobin and heme, putative mediators of toxicity from transfusions.4 5 We need better metrics for predicting red cell transfusion efficacy and toxicity. The simple expedient of fresher red cells is clearly not that metric and might be leading us to transfuse more toxic red cells (very fresh) in the most fragile patients, such as premature newborns. A new approach is clearly called for by the current data. At our centre we define fresh as <21 days of storage, and we generally never transfuse a red cell that has been stored for much less than 7-10 days, for the above reasons as well as logistics of supply. Competing interests: None declared. 1 Trivella M, Stanworth SJ, Brunskill S, Dutton P, Altman DG. Can we be certain that storage duration of transfused red blood cells does not affect patient outcomes?BMJ 2019;365:l2320. 10.1136/bmj.l2320 31186250 2 Alexander PE, Barty R, Fei Y, etal . Transfusion of fresher vs older red blood cells in hospitalized patients: a systematic review and meta-analysis. Blood 2016;127:400-10. 10.1182/blood-2015-09-670950 26626995 3 Schmidt A, Gore E, Cholette JM, etal . Oxidation reduction potential (ORP) is predictive of complications following cardiac surgery in pediatric patients[abstract]. Transfusion 2016;56(Supplement S4):20A-1A. 4 Cholette JM, Pietropaoli AP, Henrichs KF, etal . Elevated free hemoglobin and decreased haptoglobin levels are associated with adverse clinical outcomes, unfavorable physiologic measures, and altered inflammatory markers in pediatric cardiac surgery patients. Transfusion 2018;58:1631-9. 10.1111/trf.14601 29603246 5 Pietropaoli AP, Henrichs KF, Cholette JM, etal . Total plasma heme concentration increases after red blood cell transfusion and predicts mortality in critically ill medical patients. Transfusion 2019;59:2007-15. 10.1111/trf.15218 30811035 Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/ permissions LETTERS9 points -
Emergency Neonatal Transfusion in Small Hospitals
Emergency Neonatal Transfusion in Small Hospitals
applejw and 8 others reacted to Neil Blumberg for a post in a topic
9 pointsIf the unit if leukoreduced, as all red cell transfusions should be, there is no need for CMV negative in my view.9 points -
What does Lui stand for? (Freeze-thaw elution)
What does Lui stand for? (Freeze-thaw elution)
Walter Isenheim and 8 others reacted to Arno for a post in a topic
9 pointsIn this paper from 1985, "The Lui elution technique A simple and efficient method for eluting ABO antibodies c. s. FENG, K. c. KIRKLEY, c. A. EICHER, AND D. s. DE JONGH, TRANSFUSION 1985; 25:433-434.", the authors thank A. Lui. MT(ASCP)SBB, who introduced this technique to them. Therefore, I believe Lui is the name of the MT who invented this elution method.9 points -
Can I get your opinion or insight on this? Thank you.
Can I get your opinion or insight on this? Thank you.
Walter Isenheim and 8 others reacted to Malcolm Needs for a post in a topic
9 pointsIn terms of the function of the various ABO blood types, there have been a huge number of peer-reviewed papers written on the subject (and the number has exploded with the advent of COVID19). I would seriously defy anyone to keep up with all of these, but I would recommend reading pages 42-43 of Reid ME, Lomas-Francis C, Olsson ML. The Blood Group Antigen FactsBook. 3rd edition, 2012. Academic Press. ISBN: 978-0-12-415849-8. In terms of how they evolved, it is so far back now that it is anyone's guess, but slides 28 to 32 of the attached lecture may give you some idea. In Depth Lecture on The ABO and H Blood Group Systems.pptx9 points -
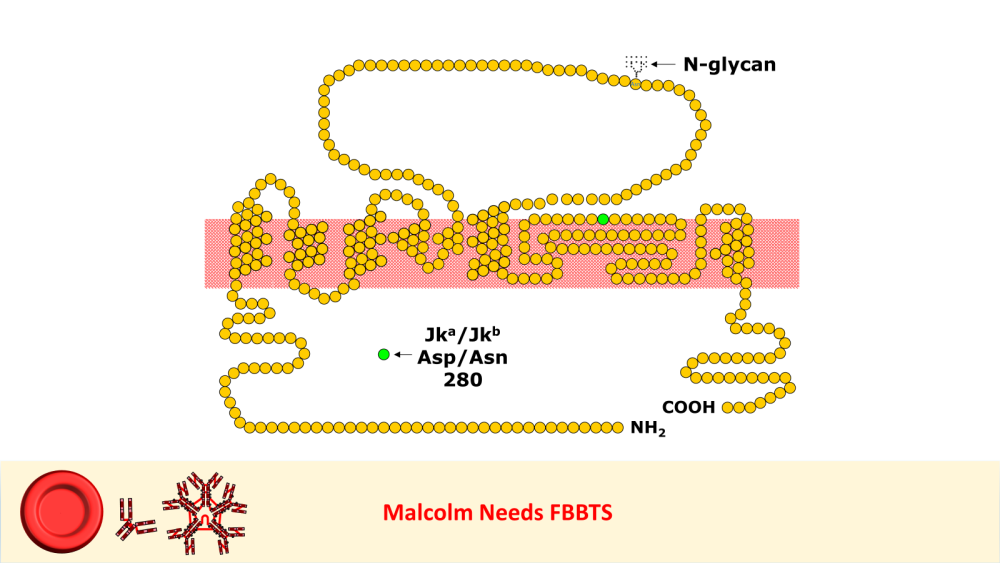
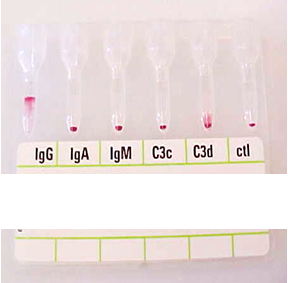
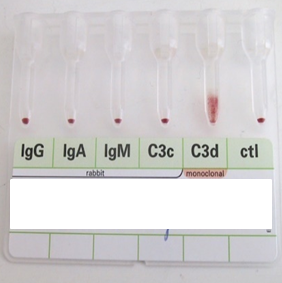
anti-Jka likes to react unpredictably?
anti-Jka likes to react unpredictably?
Arno and 7 others reacted to Malcolm Needs for a post in a topic
8 pointsI worked in Red Cell Immunohaematology for most of my 43 years before retirement, including two times at the International Blood Group Reference Laboratory (IBGRL), and for over a decade at one of the NHS Blood and Transplant Centres in London. During that time, I saw some pretty weird Kidd antibodies, but never came across an example of one that reacted with red cells with Jk(a) heterozygous expression, but not with Jk(a) homozygous expression. One such "weird" type (although I never saw one) was the extremely rare, dominant inhibitor type In(Jk), similar, but, of course, not identical to In(Lu). These red cells usually type as Jk(a-b-), but their true Kidd type can be ascertained by Adsorption and elution tests.. These red cells are also more resistant to haemolysis by 2M Urea than red cells with "normal" expression of the Kidd antigens, but less resistant to haemolysis by 2M Urea than true "amorphic" Jk(a-b-) red cells. There are approximately 14, 000 copies of the Kidd carrier molecule per red cell (quite a small number, when compared with some other carrier molecules, such as the D antigen). The amino acid residue that defines either the Jka or Jkb antigens is very close to the red cell membrane in the 4th extracellular loop but is largely “hidden” by the 3rd extracellular loop (steric hindrance). Both facts may contribute to the weak reactions between Kidd antibodies and Kidd antigens. Schematic of the Kidd carrier molecule (after Wester ES, Storry JR, Olsson ML. Characterization of Jk(a+weak): a new blood group phenotype associated with an altered JK*01 allele. Transfusion 2011; 15: 380-392. DOI: 10.1111/j.1537-2995.2010.02795.x. In this paper, Wester et al also describe weakened forms of both the Jka and the Jkb antigens, but in each case, the amino acid substitution is remote from position 280 of the mature protein. In addition, an individual with the Trp171Arg mutation with weak Jk(a) expression has produced an anti-Jk3 or anti-Jk3-like antibody, and so they may be “dangerous patients” (Whorley T, Vage S, Kosanka J, Lose SR, Sandquist AR, Copeland TR, Westhoff CM. JK alleles associated with altered Kidd antigen expression. Transfusion 2009; 41 (Suppl.): 48A-49A (abstract). Lastly (for now anyway!), most foetal red cells sensitised by maternal antibodies react only with anti-IgG, but I (and a colleague Grant Webb) have both noticed, but not published, occasions when such red cells also react with anti-C3d and, in one case, only anti-C3d (see genuine photographs below)..8 points -
CAP ALL COMMON CHECKLIST COM.04250
CAP ALL COMMON CHECKLIST COM.04250
SBBSue and 7 others reacted to Malcolm Needs for a post in a topic
8 pointsSo, this PROVES that CAP do not know the A from their elbow. ALL Blood Transfusion Reference Laboratory Staff, not to mention MOST Blood Transfusion Hospital Laboratory Staff KNOW that not all antibodies can, by any means, be detected by ALL serological techniques (saline, albumin, enzyme, LISS, IAT, inhibition tests, recombinant blood group proteins, etc), let alone by ALL technologies (glass, tube, plastic tube, liquid phase microtitre plates, solid phase microtitre plates, column technologies, etc), BUT THOSE WHO RUN CAP KNOW BETTER THAN EVERYONE. They should be thoroughly ashamed of themselves, and go back to kindergarten.8 points -
Antigen typing during pregnancy
Antigen typing during pregnancy
Ensis01 and 7 others reacted to John C. Staley for a post in a topic
8 pointsI've never heard of that. While I can understand the rationale, I'm afraid that if there was enough of a fetal bleed to impact antigen testing mom there are bigger problems than just getting the antigen type right. Just my thoughts.8 points -
Repeat of donor Antigen typing
Repeat of donor Antigen typing
David Saikin and 7 others reacted to NicolePCanada for a post in a topic
8 pointsWe don't recheck antigen typings here in our hospital in Canada. The typings that have been performed at Canadian Blood Services, are embedded in the barcode on the bag, with all negatives printed on the End User Label. Every unit is antigen typed for K so if it isn't printed on the bag the unit is K Pos. Antigen typings we do are all linked to the unit through barcode. The reason of, "We were typing a lot of units and may have mixed them up", is not acceptable in a blood bank setting. Go work in a different department if you can't organize yourself. Anyway, there is also a full gel or whatever you use crossmatch at the end of that phenotyping, as long as the antibody is reacting, an anomaly could be discovered there. You have to have a little faith that people before you are doing their job properly, or you can cause yourself a lot of undue stress.8 points -
How often must staff sign policies?
How often must staff sign policies?
TreeMoss and 7 others reacted to Neil Blumberg for a post in a topic
8 pointsI realize this is "fighting city hall" but is there a more useless requirement than having everyone review and sign off on procedures that haven't changed one iota? In our laboratory, this is many hundreds of procedures (including the one on how to write a a procedure :). Bureaucratic make work of no value whatever. An unfortunate example of the administrative/legal mindset versus the scientific/clinical mindset in our society. Probably an early small sign of the coming end of our civilization when non-productive work receives such priority. Seriously.8 points -
why 3 months?
why 3 months?
Yanxia and 7 others reacted to Malcolm Needs for a post in a topic
8 pointsThe three months was chosen following a paper written by Laine EP, Leger RM, Arndt PA, Calhoun L, Garratty G, Petz LD. (In vitro studies of the impact of transfusion on the detection of alloantibodies after autoadsorption. Transfusion 2000; 40 1384-1387. DOI: 10.1046/j.1537-2995.2000.40111384.x.) that showed that red cells that had been transfused (or entered the circulation via a feto-maternal haemorrhage could adsorb out weak alloantibodies for up to three months in a patient with AIHA. This in vivo adsorption would, of course, also apply to individuals who did not have AIHA, but could lead to a secondary stimulation, leading to a stronger antibody (higher titre and higher concentration per mL of plasma), if the alloantibody was "missed" in the antibody screen and/or cross-match, particularly as it is unlikely that the full phenotype of the transfused (or foetal) red cells would be known.8 points -
Storing Saline Cubes
Storing Saline Cubes
jshepherd and 7 others reacted to jayinsat for a post in a topic
8 pointsWe had an over zealous infection control team (made up of 100% nurses) come to our lab last year making the same demand. We told them, in essence, we will not comply because the risk of injury from handling those containers were greater than the risk they were trying to alleviate. Furthermore, the risk of accidently confusing saline with formalin, whose containers look exactly alike, was to high when removing from the cardboard containers. In addition to that, we told them the man hours required to keep up with that would require additional FTE's, which would not be approved. They conceded and we continued on, business as usual. TJC does not really inspect labs that are CAP, AABB, or CLIA certified. Those organizations understand the logistics of the cubes and do not have a problem with it. Most infection control officers are nurses and think from the nursing perspective only.8 points -
A 10month A pos baby with anti- A
A 10month A pos baby with anti- A
Mabel Adams and 7 others reacted to Arno for a post in a topic
8 pointsHere is an interesting paper showing that antibodies to red cell/platelet... may be transmitted via breast milk indeed, causing prolonged HDFN. Milk contains mostly IgA but IgM and IgG may be present of course and IgGs can cross the different barriers up to blood circulation (not on the same model - not actively - as the placenta though). The surprizing part here is the mother and baby are group A, A antigen is ubiquitous so the anti-A titer in breast milk should high enough to interfer with reverse group despite the adsorption of anti-A on various tissues. https://pubmed.ncbi.nlm.nih.gov/30720868/8 points -
Use of plastic tubes for tube testing
Use of plastic tubes for tube testing
AuntiS and 7 others reacted to Sonya Martinez for a post in a topic
8 pointsI did check our IFU and they did specifically state to use glass tubes. No more arguments.8 points -
Why irradiate liquid plasma when RBCs for trauma patients aren't irradiated?
Why irradiate liquid plasma when RBCs for trauma patients aren't irradiated?
MinerJ and 7 others reacted to Neil Blumberg for a post in a topic
8 pointsThe mechanisms of what have been termed TRALI (actually a subset of acute lung injury/acute respiratory distress syndrome) and TACO (actually something very common, congestive heart failure) have been widely misunderstood due to unjustified assumptions/dogma. There are many biologic mediators other than antibodies that can cause lung injury after venous infusion which directly subjects the lung vascular endothelium to these mediators (antibodies, activated cells, lipids, mediators such as sCD40L, DNA/histones). Likewise there are many mediators that can cause or exacerbate cardiac failure after venous infusion (inflammatory mediators, excess volume). Cardiac failure is not just volume overload, but can be caused by fever, inflammatory cytokines and vascular/myocardial muscle dysfunction. The notion that these are distinct entities is also at variance with clinical experience. Many patients have signs of both cardiac failure and pulmonary failure simultaneously. So the definitions and pathophysiology used in reviews and texts are lacking in validity and just plain oversimplified and wrong, in my view. There are compelling data to support these iconoclastic contentions for TRALI, and some for TACO. Most germane (see attachment), when we introduced universal leukoreduction, we saw a sustained 83% drop in reports of TRALI and 50% in TACO over the following years. This suggests that white cells/DNA/histones play a role in causing lung and heart inflammation and dysfunction. This clinical observation was confirmed in animal studies from Denisa Wagner's lab at Harvard demonstrating that neutrophil extracellular traps (NETS) infused intravenously can cause acute lung injury (see attachment). To me these observations are convincing evidence that leukoreduction alters cardiorespiratory injury and failure post-transfusion and represents one of the strongest arguments for universal leukoreduction. Needless to say, this challenge to dogma has been ignored by the transfusion medicine community which continues, at least in the USA, to infuse deadly white cells and their degradation products (free DNA/histones) to patients, one of the great tragedies of the last 20 years in the USA blood bank field. We got this entirely wrong and tens of thousands of patients have probably died unnecessarily due to complications of non-leukoreduced transfusions. ULR TRALI TACO PMC version.pdf NETS and TRALI Wagner 2012.pdf8 points -
Tube Antibody Titers: Yes or No to Enhancement?
Tube Antibody Titers: Yes or No to Enhancement?
Malcolm Needs and 7 others reacted to exlimey for a post in a topic
8 pointsAn excellent discussion point. I think many others have similar questions and concerns. The have been several other threads on this forum with similar subject matter. As an Old Fart, I feel obliged to spout some (un-referenced) history. Most of the original work on clinical significance of antibodies in pregnancies was done in the absence of potentiators and definitely before the use of (semi)automated test systems. I think it was a "saline-IAT" using 22% albumin (BSA) as a diluent. Most of those antibodies were anti-D, for obvious reasons. There's not much out there in the literature in terms of controlled or organized studies regarding other specificities. There are a fair number of one-of-a-kind case studies, but most of the stuff is retrospective analysis of data. Basically, other than anti-D, nobody really knows what an antibody titer means, but as Ensis01 suggests, detecting a change in titer (increase) may be more important. In an era when basic tube shaking is going away, it only makes sense (we have no other option) to convert to the new techniques and equipment, but I suspect that it has the potential to further confuse an issue which already has enough confusion to (dis)satisfy everyone. I don't envy anyone handling this hairball. As a last thought...the high-powered potentiators (and techniques) used today don't reflect what's going on in vivo. Arguably, if one ignored the 22% BSA diluent, the saline-IAT is a better mimic of the in vivo scenario.8 points -
IAT & Ab ID
IAT & Ab ID
Kelly Guenthner and 6 others reacted to Malcolm Needs for a post in a topic
7 pointsColumn agglutination technology is an excellent technique, but does have a tendency to detect antibodies that react at temperatures well below 37oC, even after fairly prolonged incubation at 37oC. However, the fact that the blood group, including the "reverse grouping" is clear of atypical agglutination suggests that this may not necessarily be the case for this patient. Just to be on the safe side though, and if you can, I would either treat the plasma from the sample with rabbit erythrocyte stroma (which will adsorb out most "cold" agglutinins), treat the plasma with 0.01M dithiothreitol (which will denature the J-chains of IgM molecules, meaning that, although they can still sensitise the red cells, they are no longer able to agglutinate the red cells) or, and my personal favourite, is to pre-warm the plasma and red cells to 37oC before mixing, perform the IAT at strictly 37oC in glass tubes, wash with saline warmed to 37oC and use monospecific AHG. If any, or all, of these techniques lead to negative results, the chances are that the antibody is a clinically insignificant "cold" IgM antibody, such as an auto-anti-HI (given that the patient is group A, and the test cells are all group O).. Failing the above, send a sample to a red cell reference laboratory. I hope that helps a little bit.7 points -
New Blood Group System.
New Blood Group System.
Sherif Abd El Monem and 6 others reacted to Malcolm Needs for a post in a topic
7 pointsA research team led by NHS Blood and Transplant scientists based in Bristol, at NHSBT’s International Blood Group Reference Laboratory (IBGRL), and supported by colleagues at the University of Bristol, has discovered a new blood group, MAL. 🙌 🩸 They identified the genetic background of the previously known but mysterious AnWj blood group antigen, thus allowing identification and treatment of rare patients lacking this blood group. Louise Tilley, Senior Research Scientist, IBGRL Red Cell Reference at NHS Blood and Transplant, said: “The genetic background of AnWj has been a mystery for more than 50 years, and one which I personally have been trying to resolve for almost 20 years of my career. It represents a huge achievement, and the culmination of a long team effort, to finally establish this new blood group system and be able to offer the best care to rare, but important, patients." hashtag#NHSBT hashtag#GiveBlood hashtag#SaveLive hashtag#NHSCareers Activate to view larger image,7 points -
Letting tube sit to catch rouleaux or cold?
Letting tube sit to catch rouleaux or cold?
Kelly Guenthner and 6 others reacted to Malcolm Needs for a post in a topic
7 pointsIf the test is showing no immediate rouleaux or agglutination due to a "cold reacting" antibody, I'm afraid that I am at a loss to see why anyone would leave the tests to see whether one or the other develops. Rouleaux is clinically insignificant in terms of blood transfusion, and ditto "cold reacting" antibodies, unless they are of wide thermal amplitude (in which case, there would almost certainly be agglutination visible straight away. Why waste reagents and expensive technician's time investigating a clinically insignificant phenomenon?7 points -
How not to miss a weak reaction
How not to miss a weak reaction
Kelly Guenthner and 6 others reacted to Malcolm Needs for a post in a topic
7 pointsIt sounds to me like you are doing everything that you should do, without either over-shaking the tube, or over-reading the contents. I am extremely glad that you are not using a microscope, as, if you did, you would almost certainly see the odd couple of red cells "kissing each other", even if they have been incubated in isotonic saline. The other thing is (and I speak with some 43 years of working in blood group serology) if the reactions in the tube are THAT weak, the chances of any atypical alloantibody that you might miss being clinically significant are absolutely minute. If you are still worried, however, get a more experienced worker to read your tests as well, until you feel confident. That is how I learned when I started. I wish you the best of luck in your future career.7 points -
Antigen typing during pregnancy
Antigen typing during pregnancy
Kelly Guenthner and 6 others reacted to Neil Blumberg for a post in a topic
7 pointsNot a sensible approach in my opinion. No real chance of mistyping due to fetal bleed. At very least, you'd see a mixed field if there were a fetal bleed with a different type. So get rid of this requirement in my view.7 points -
Prenatal Antibody Titers
Prenatal Antibody Titers
SbbPerson and 6 others reacted to Malcolm Needs for a post in a topic
7 pointsWell, the first thing to say is that red cells CANNOT be either homozygous or heterozygous (or, come to that, hemizygous). These terms apply ONLY to genes, and red cells do not contain a nucleus. The antigens can only be described as, at best, "homozygous", "heterozygous" or "hemizygous" expression, or, alternatively, "double" or "single dose" expression. Then, it HAS to be accepted that, unless the maternal antibody is an autoantibody, it must be an alloantibody (or, possibly, an isoantibody), which means that to mimic the state of the foetal red cells, the red cells used to titrate the antibody MUST have a "single dose" expression. However, that in itself presupposes that the foetal red cell antigens are all expressed at the same time, which we know is untrue (just look at the A, B and H antigens as an obvious example, but also the Kell antigens that are expressed much earlier than are the Rh antigens) or are ONLY expressed on foetal red cells, as opposed to other tissues (such as on the placental cells, which have, in some cases, been proved to adsorb the maternal antibodies). Then, there is the fact that not all antibodies can be detected by all techniques. This is why Reference Laboratories SHOULD have more than one technology available (and their workers should be provably competent in these techniques. However, even then, not all techniques can predict the severity or otherwise of HDFN. For example, antibodies within the Indian Blood Group System always show that they can cause severe HDFN by certain techniques, such as MMA, but they don't! There is also the fact that the immunoglobulins may be IgM, IgA, IgG1, IgG2, IgG3 and IgG4 (to mention just a few), and I have yet to come across, or read about, an IgG4 immunoglobulin causing HDFN. So, my answer is that there is a HUGE amount of knowledge known about the various antibody specificities, their titres, the expression of their cognate antigen, etc, etc, that there CANNOT be a single answer to your excellent question, but that the best thing that can be done is to read around the subject - and read around the subject from every source available - not just from a single country. OKAY THEN, RIP ME APART!!!!!!!!!!!7 points -
Incompatible Blood
Incompatible Blood
Yanxia and 6 others reacted to AMcCord for a post in a topic
7 pointsAgree! Save the life first. Our medical director would likely order at least one DAT the next day, possibly for additional days, to monitor. Anti-E is generally relative benign (though I have seen one patient who had an acute hemolytic reaction), We might also monitor plasma Hgb or haptoglobin, depending on the antibody involved.7 points -
Platelet Compatibility
Platelet Compatibility
ffriesen and 6 others reacted to jayinsat for a post in a topic
7 points@Neil Blumberg, I wish we had you at all of our facilities to educate our medical staff. Sadly, convincing Hematologists and Oncologists (at least here in America) that it is better to postpone platelet transfusions than give ABO incompatible platelets is, more often than not, rejected, especially in light of the fact that many patients are having to wait because of lack of platelet inventory to begin with. What we really need is a push for better transfusion therapy education in medical school. Along with this, continuing education for practitioners needs to become a priority. It is, however, quite difficult to get time with these practitioners. Even when we convince our laboratory medical directors to advocate for these issues, in my experience, clinicians rarely change. All that said to say, in the "trenches," the practice will likely continue to prioritize inventory over safety.7 points -
Platelet Compatibility
Platelet Compatibility
CARMEN DELGADO and 6 others reacted to Neil Blumberg for a post in a topic
7 pointsI should add the good news is that when one starts prioritizing ABO identical platelets over inventory management, one reduces the platelet transfusions needed by perhaps 50%. So our platelet shortages will disappear in large part if we stick with ABO identical as much as possible. See attached randomized trial from eons ago :). ABO identical reduces transfusion reactions as well, HLA and rbc alloimmunization. Not to mention decreasing bleeding and mortality. ABO randomized trial UR european j haematology 1993 copy.pdf ABO plt tx revisited cumulative effects.pdf Platelet transfusion worsens ICH Stroke 2020 copy.pdf7 points -
Platelet Compatibility
Platelet Compatibility
CARMEN DELGADO and 6 others reacted to Neil Blumberg for a post in a topic
7 pointsAnother point. Since group O whole blood has proven as safe or even safer than typical component therapy (A platelets, A or AB plasma) in massive transfusion of trauma patients, perhaps group O low titer platelets would be safer than group A or B platelets for an AB patient :)? No one knows, but worth considering. The big problem is probably giving non-O platelets to O patients. There is evidence this increases bleeding and mortality. Just like red cells, only O platelets for O recipients is a good practice. The AB patient may be less of a problem, since giving some small amount of antibody may be less dangerous. A risk of hemolytic reaction of about 1 in 700 or so. The risk of mortality in transfusing an O patient with A platelets is probably 1 in 5 (see attached). ABO incompatible platelets intracranial bleeding 2021.pdf ABO plasma incompatible platelets and hemolytic reactions.pdf7 points -
Platelet Compatibility
Platelet Compatibility
TreeMoss and 6 others reacted to Neil Blumberg for a post in a topic
7 points"Since AB+ people are considered the "universal recipient" , we give them any type platelets, usually starting with the one with the closest out date. " I grant you that this is widely shared idea in our field for decades. It is also seriously wrong. It prioritizes inventory management over patient wellbeing. Our approach to ABO and platelets is distinctly different from ABO and red cells with no rational basis. Antibody and complement destroy red cells and platelets equally well. The only difference is that instead of free hemoglobin being released, it's mediators such as VEGF, IL-6 and other platelet pro-inflammatory, immunomodulatory and pro-thrombotic granule contents are released. ABO mismatched platelet transfusions at least double the refractoriness rate in repetitively transfused patients (see attached for references), and actually increase bleeding and mortality. The answer to the question is ABO identical is by far most effective and safest. If you have to give ABO mismatched, there is probably no good answer other than washed/volume depleted O's, A's or B's, where most of the incompatible plasma is removed. If that's not possible, postponing platelet transfusion until ABO identical is available when feasible, giving half doses of ABO identical if two patients need the one available unit, etc. are also reasonable. Sadly, ABO mismatched platelets are probably worse than no platelets at all. They provide little or no hemostatic benefit and increased risks of bleeding, organ injury and death for the patient. If I were the attending physician, I would generally give no platelets if ABO identical or washed O's weren't available in a stable, non-bleeding patient with a count of over 5,000. The good news is we can improve outcomes by just doing what we do for red cells. Do not transfuse ABO incompatible antigen or antibody. It's bad for red cells, platelets and endothelial cells, all of which have complement and Fc receptors that bind immune complexes, and all of which bear ABO antigens on their surfaces. Carr ABO mismatched refractoriness copy.pdf ABO story expanded.docx ABO endothelial cell paper.docx NEJMc2034764 copy.pdf NEJMc2034764_appendix copy.pdf7 points -
Antibody stimulation by antigen negative blood?
Antibody stimulation by antigen negative blood?
SbbPerson and 6 others reacted to RichU for a post in a topic
7 pointsI used this case study as part of my Higher Specialist Diploma in Blood Transfusion. The IBMS have asked if I would like to give my PowerPoint presentation ('What the f?') at the 2023 Congress. Thank you to all the contributors - I will certainly big up PathLabTalk if I do get to do it. Rich7 points -
Is it illegal to lie about your genomic blood type?
Is it illegal to lie about your genomic blood type?
AMcCord and 6 others reacted to Malcolm Needs for a post in a topic
7 pointsIt is all over the place, to be honest. It is Caucasian, rather than caucasian, It is group O, D Positive, and group A, D Positive, rather than either group O Positive or group A Positive (see the early editions of Peter Issitt's book). It is Oh (with a subscript "h"), and not "Bombay". The FUT1 gene, or, rather, the lack of a functional gene through various different genetic mutations, leads to the "Oh" phenotype, but this should NOT be called the "Bombay phenotype". Although this phenotype was first described by Bhende YM, Deshpande CK, Bhatia HM, Sanger R, Race RR, Morgan WTJ, Watkins WM. A “new” blood-group character related to the ABO system. Lancet 1952; i: 903-904. DOI: 10.1016/S0140-6736(52)92356-8, Another example of the Oh phenotype can be seen in the rare recessive condition, Leukocyte Adhesion Deficiency Type II where, to all intents and purposes, the patient will have a normal H gene, and yet the red cells are of the Oh phenotype, and anti-H can be found in the plasma. the phenotype has been identified in many different parts of the world (and is not just confined to mutations in India or even Asia (Hidalgo A, Ma S, Peired AJ, Weiss LA, Cunningham-Rundles C, Frenette PS. Insights into leukocyte adhesion deficiency type 2 from a novel mutation in the GDP-fucose transporter gene. Blood 2003; 101: 1705-1712. DOI: 10.1182/blood-2002-09-2840). The other thing is, of course, that "Bombay" no longer exists - it is now Mumbai! I APOLOGISE FOR BEING A COMPLETE PEDANT!7 points -
Dream equipment/products/supplies?
Dream equipment/products/supplies?
BldBnker and 6 others reacted to Ensis01 for a post in a topic
7 pointsDoes acquiring more good blood banking staff count?7 points -
Patient with WAA unable to determine ABO & Rh type
Patient with WAA unable to determine ABO & Rh type
applejw and 6 others reacted to Malcolm Needs for a post in a topic
7 pointsWith all due respect, if you do not trust the results given to you by your IRL, why did you send samples to them in the first place? The other thing is that the term "least incompatible" has been "rubbished" by LaTwrie Petz almost 20 years ago now (and you can't get much better than him!) - see Petz LD. "Least incompatible" units for transfusion in autoimmune hemolytic anemia: should we eliminate this meaningless term? A commentary for clinicians and transfusion medicine professionals. Transfusion 2003; 43(11): 1503-1507. DOI: 10/1046/j.1537-2995.2003.00583.x. I apologise if this reads as I am being personally rude to you; that is DEFINITELY NOT my intention.7 points -
Positive Baby DAT and Fetal Screen
Positive Baby DAT and Fetal Screen
AMcCord and 6 others reacted to jayinsat for a post in a topic
7 pointsDanielle, I love the lights. When i'm feeling stressed, I come here and break em. If you haven't noticed, move over them with your mouse and they break with the most refreshing sound of glass breakage. I hope you all don't think less of me now...7 points -
Pre-Transfusion Two-Blood Group Policy
Pre-Transfusion Two-Blood Group Policy
David Saikin and 6 others reacted to John C. Staley for a post in a topic
7 pointsAs Joanne mentioned above, no system is fool proof and there are lots of creative, inventive fools to prove it. Keep your system as simple as possible which should minimize the need for creative people to find ways around it. Now to your question, does it actually help prevent problems? Probably a few but certainly not all! I've seen people become lax in their diligence when they assume they are protected by the system. They seem to assume that if they make a mistake someone down the line with catch it. This is something to be avoided if possible. The only way that I know of to prevent this type of mind set from developing is through education and convincing everyone involved in the process that their step is critical and by keeping it simple they will be more likely to perform their step as instructed.7 points -
Newly detected anti-D
Newly detected anti-D
SbbPerson and 6 others reacted to carolyn swickard for a post in a topic
7 pointsWhat about RH pos plasma products or platelets? Though they don't tend to cause an anti-D, they can "spike" one that dropped below detectable levels, I believe. And that far back, if any platelet concentrates were given, they would have had more RBC exposure than they do now with platelet pheresis units. Just a thought.7 points -
Tube Antibody Titers: Yes or No to Enhancement?
Tube Antibody Titers: Yes or No to Enhancement?
Malcolm Needs and 6 others reacted to Ensis01 for a post in a topic
7 pointsMy experience is that the BB reports out the antibody identification. Never the reactivity! If a titer is ordered the only thing reported is the titer or “too weak to titer”. As the rise in titer is the most relevant result, consistency in method and technique is very important, both within your hospital system and the reference lab you use. Physicians are interested in your results not the process. Keep that simple. If they have questions your Medical Director can enlighten them.7 points