Leaderboard
-
Points8,971Posts
-
 Points279Posts
Points279Posts -
-
Points1,581Posts
Popular Content
Showing content with the highest reputation since 06/18/2025 in all areas
-
Plasma transfusions
Plasma transfusions
psykobillys and 4 others reacted to Neil Blumberg for a post in a topic
5 pointsAlso, were any of the transfused units antigen positive? This is the quickest way to get a negative indirect antiglobulin test ;).5 points -
Gel vs tube for DARA patients
Gel vs tube for DARA patients
Yanxia and 3 others reacted to jtemple for a post in a topic
4 pointsWhat? All this time I have been using the wrong stuff! Ha! SPELL CHECK IS NOT YOUR FRIEND! 🤣🤣🤣🤣4 points -
Maternal alloantibody, not detected in baby - how long for antigen negative units
Maternal alloantibody, not detected in baby - how long for antigen negative units
Neil Blumberg and 2 others reacted to John C. Staley for a post in a topic
3 pointsCouple of questions for clarification. What is the specificity of the known Alloantibody? "Baby is born and our testing shows negative antibody screen." Was this AB screen done on mom or baby? If on the baby, was a current ab screen performed on mom and if so what was the results? Was a DAT performed on the baby? If so, what was the result? If not, why not? Thanks3 points -
Gel vs tube for DARA patients
Gel vs tube for DARA patients
Yanxia and 2 others reacted to Malcolm Needs for a post in a topic
3 pointsUm, sorry Jason, but I think you mean Dithiothreitol (DTT), rather than Dichlorodiphenyltrichloroethane (DDT)!!!!!!!3 points -
A little about me
A little about me
donellda and 2 others reacted to Cliff for a post in a topic
3 pointsI rarely post about me, or my family. I was raised by a single mom from 2 until I was about 10. That was probably really hard for her in the 60s. Whenever I ask her, she just says it wasn't so hard. We had a lot of roommates to help with the cost of apartments, one was Georganne (Giorgi Baino Sr.). She seemed to be around the longest. I really liked her, she was like an aunt to me. Maybe 10 or more years ago, we got back in touch - Sadly, just on Facebook. I am going to try to have a video chat with her to catch up. Anyhow, it seems my mom wrote a poem about me when I was a kid, and Georganne has always played guitar. The song was about me riding bikes, I never heard it until yesterday. For those that know me, you know I've been a cyclist (avid at times) for the last 15 years. She put the poem to music and sent it to me yesterday. I cried. Here it is if you're interested. I cried.3 points -
Maternal alloantibody, not detected in baby - how long for antigen negative units
Maternal alloantibody, not detected in baby - how long for antigen negative units
Darin and one other reacted to Neil Blumberg for a post in a topic
2 pointsThanks Malcolm. Never say never :).2 points -
Maternal alloantibody, not detected in baby - how long for antigen negative units
Maternal alloantibody, not detected in baby - how long for antigen negative units
Yanxia and one other reacted to Malcolm Needs for a post in a topic
2 pointsArndt PA, Garratty G, Marfoe RA, Zeger GD. An acute haemolytic transfusion reaction caused by an anti-P1 that reacted at 37 degrees C. Transfusion 1998; 38(4): 373-377. DOI: 10.1046/j.1537-2995.1998.38498257376.x. Smith D, Aye T, Er LS, Nester T, Delaney M. Acute hemolytic transfusion reaction due to anti-P1: a case report and review of institutional experience. Transfus Med Hemother 2019; 46: 381-384. Published online as DOI: 10.1159/000490897. Irani MS, Figueroa D, Savage G. Acute hemolytic transfusion reaction due to anti-Leb. Transfusion 2015; 55: 2486-2488. DOI: 10.1111/trf.13178. Delk AA, Gammon RR, Alvarez H, Benitez N, Bright F, A hemolytic transfusion reaction caused by an unexpected Leb antibody. Laboratory Medicine 2021; 52: 303-306. DOI: 10.1093/labmed/lmaa070.2 points -
K Neg units requested for patient with autoimmune aplastic anemia
K Neg units requested for patient with autoimmune aplastic anemia
Neil Blumberg and one other reacted to kjmiller for a post in a topic
2 pointsGood morning, Malcom. Thanks for your reply- she is 61 yrs old and KNeg. I agree also with your approach, but we also have lots of patients with chronic anemias on transfusion support for whom we aren't giving K Neg. Maybe the dr. is just being more proactive in this case.2 points -
Maternal alloantibody, not detected in baby - how long for antigen negative units
For acquired maternal IgG antibodies (which may also be transferred postnatally through breast milk), assessing the antibody specificity (AbS) in the newborn, as previously mentioned, appears to be a reasonable approach. In addition, the Direct Antiglobulin Test (DAT) remains key, and performing an elution is important (even if the DAT result is negative). In your case, the negative DAT suggests that either the anti-N antibody did not cross the placenta, possibly due to being a naturally occurring IgM, and/or the baby is N-negative.2 points
-
Maternal alloantibody, not detected in baby - how long for antigen negative units
Maternal alloantibody, not detected in baby - how long for antigen negative units
Yanxia and one other reacted to Malcolm Needs for a post in a topic
2 pointsIt is incredibly rare for anti-N to be an alloantibody, unless the individual is M+N-, and also S-s-U-. This is because the amino acids that characterise the N antigen on the Glycophorin A molecule (leucine, serine, threonine, threonine, glutamic acid) are identical to the amino acids that characterise the 'N' antigen on the Glycophorin B molecule. Is the lady of Black ethnicity by any chance? If not, to be N Negative AND 'N' Negative would be almost unique. This suggests to me that the anti-N reported to be in the maternal circulation by the other hospital may well have been an auto-antibody, and would almost certainly be sub-clinical in its significance. In such a case, I would not bother with performing genotyping of the baby's N type. However, as far as Rh, K, etc, I would certainly suggest that antigen negative blood is given to the baby, and I certainly WOULD perform foetal genotyping (see my answer to Cliff above).2 points -
Maternal alloantibody, not detected in baby - how long for antigen negative units
Maternal alloantibody, not detected in baby - how long for antigen negative units
Yanxia and one other reacted to Malcolm Needs for a post in a topic
2 pointsThe reason I said this (and I admit that I am being more than a little "Reference Laboratory Pedantic here) is because a very good friend of mine (Edmond Lee, who used to work at the NHSBT-North London Centre at Colindale, with such luminaries as Prof Dame Marcela Contreras, Dr Mahes de Silva and Martin Redman, amongst others, who described a case where the bay of a woman with an extremely strong anti-K,, where the baby's foetal K antigens were blocked by the maternal anti-K, and so tested as negative (Lee E, Redman M, Owen I. Blocking of fetal K antigens on RBC by maternal anti-K. Transfus Med 2009; 19(3):139-40. doi: 10.1111/j.1365-3148.2009.00917.x. Later, he reported the same sort of thing with a maternal anti-Fy(a) (Lee E, Cantwell C, Muyibi KO, Modasia R, Rowley M, New H. Blocking phenomenon occurs with murine monoclonal antibodies (anti-Fya) in a neonate with a positive direct antiglobulin test due to maternal anti-Fy(a). Blood Transfus 2015; 13: 672-674. doi: 10.2450/2015.0232-14. Obviously, in both these cases, the maternal antibody was easily detectable, so not the same as the case being described by BullDawgPath, and, in both cases, the baby's DAT was positive, BUT, in both cases, antigen negative blood was required by the baby.2 points -
Maternal alloantibody, not detected in baby - how long for antigen negative units
Maternal alloantibody, not detected in baby - how long for antigen negative units
John C. Staley and one other reacted to Malcolm Needs for a post in a topic
2 pointsAll great questions, but I would also ask, what is the baby's Hb/Hct requiring a transfusion, and why not test the baby's DNA for the gene encoding the antigen cognate to the maternal antibody?2 points -
Plasma transfusions
Plasma transfusions
bblover and one other reacted to Malcolm Needs for a post in a topic
2 pointsAGREED - and killing the patient in some circumstances!!!!!!!!!!!!!!!!!!2 points -
Plasma transfusions
Plasma transfusions
Yanxia and one other reacted to John C. Staley for a post in a topic
2 pointsHow many units of uncrossmatched blood did they receive? How active were they bleeding? How much later did the other hospital preform their own T&S? Inquiring minds want to know!!! 😉2 points -
Plasma transfusions
Plasma transfusions
AuntiS and one other reacted to Malcolm Needs for a post in a topic
2 pointsI agree Darin, it is almost certainly a dilutional effect, BUT, it could also be the effect of a soluble antigen (obviously not within the Duffy Blood Group System). If the antibody had a specificity within, for example, the Lewis Blood Group System, or the Chido/Rodgers Blood Group System, the antigen in the plasma could well adsorb out the circulating antibody. That having been said, this explanation is FAR less likely than your suggestion of the dilutional effect.2 points -
Plasma transfusions
2 pointsWe did a Plasma Exchange recently on a patient 5 days in a row with approximately 10-12 units of FFP each time. On day 3, the band expired and we retested the new sample to find the previous antibody had disappeared. It's a dilutional effect where the titer is dropped below detectable levels.2 points
-
Quote of the day: 07/17/2025
Quote of the day: 07/17/2025
Darin reacted to Inspiration Bot for a post in a topic
1 pointIf we shall take the good we find, asking no questions, we shall have heaping measures. ~ Ralph Waldo Emerson1 point -
TRM.31400 Reagent red cell QC
1 pointYes, it's now Werfen (haven't been too happy with that change). We utilize the Capture-R NEG/POS control Serum and monitor reaction strength. This approach was selected to ensure quality and exceed the "Manufacturer's Recommendation" which, as you stated, will be decided on a lab by lab basis.1 point
-
TRM.31400 Reagent red cell QC
1 pointPresuming that "Panocell III" is the Werfen 3-cell screen, for which antigens are you testing and how did you decide on those ? Just curious. I think everyone may approach this nutmeg a little differently.1 point
-
Maternal alloantibody, not detected in baby - how long for antigen negative units
Maternal alloantibody, not detected in baby - how long for antigen negative units
Darin reacted to Malcolm Needs for a post in a topic
1 pointLove that!1 point -
Maternal alloantibody, not detected in baby - how long for antigen negative units
Maternal alloantibody, not detected in baby - how long for antigen negative units
Malcolm Needs reacted to Neil Blumberg for a post in a topic
1 point"I'm sorry Neil, but Geoff Daniels quotes some HTR's caused by anti-N reacting at 37oC," These are, if I remember correctly, fairly ancient reports and I have never seen nor heard of a case of hemolytic transfusion reaction or HDFN due to anti-N despite having had hundreds of patients with anti-N in our service over the last half century. I've never heard of anyone else seeing one. So this is very possibly a case of old reports of hemolysis due to other causes (undetected antibodies for example). Methodology for antibody detection in the 1940s and 1950s, and even 1960s, was significantly less sensitive and accurate than currently. There are reports mentioned in Mollison and other comprehensive texts such as Daniels of hemolytic reactions due to antibodies (e.g., anti-P1, anti-Leb, etc.) that have never been reported in modern literature (the last 30-40 years). This makes me suspicious that these old reports are mistaken as to the cause of hemolysis. If the mother has an anti-N and the infant is not hemolyzing, and the antibody is undetectable I would not transfuse N negative blood. If the infant is hemolyzing, that is another story, obviously. A positive DAT, hemolysis and anti-N in the mother would dictate prudence and transfusing N negative blood. But I will stand by my original comment, which is that anti-N almost never causes clinically significant hemolysis in transfusion recipients nor in affected fetuses. Absent clinical and laboratory evidence for anti-N causing the infant's anemia, there is no reason to transfuse N negative blood when the antibody is not detectable in the fetus/infant.1 point -
ChemLabTalk: Biuret method
ChemLabTalk: Biuret method
Malcolm Needs reacted to Cliff for a post in a topic
1 pointThat's OK. Pass (yay!) or fail, either way, it's always a learning opportunity.1 point -
Maternal alloantibody, not detected in baby - how long for antigen negative units
Maternal alloantibody, not detected in baby - how long for antigen negative units
Yanxia reacted to Malcolm Needs for a post in a topic
1 pointI'm sorry Neil, but Geoff Daniels quotes some HTR's caused by anti-N reacting at 37oC, and one case of mild HDFN in a M+ N+ baby, where the mother was M+ N-, S-, s- Uvar, in the third edition of his book, Human Blood Groups.1 point -
Maternal alloantibody, not detected in baby - how long for antigen negative units
Maternal alloantibody, not detected in baby - how long for antigen negative units
John C. Staley reacted to Neil Blumberg for a post in a topic
1 pointAnti-N does not cause hemolytic Transfusion reactions nor hemolytic disease of the newborn and fetus. So I would not give N negative blood in general, nor if the cross match is negative.1 point -
Maternal alloantibody, not detected in baby - how long for antigen negative units
Maternal alloantibody, not detected in baby - how long for antigen negative units
Yanxia reacted to Malcolm Needs for a post in a topic
1 pointIn that case, I would consider a genotype, as getting hold of M+ N-, S-s-U- fresh units is not going to be easy. That having been said, as you say yourself, anti-N is rarely clinically significant and, if it is not detectable in either the maternal circulation, or in the baby's circulation, I wouldn't worry too much about giving M+, N-, S-s-U- blood. BEAR IN MIND THOUGH, THIS WILL BE A CLINICAL DECISION, AND I AM NOT, AND NEVER HAVE BEEN, MEDICALLY QUALIFIED.1 point -
Maternal alloantibody, not detected in baby - how long for antigen negative units
Maternal alloantibody, not detected in baby - how long for antigen negative units
Neil Blumberg reacted to Cliff for a post in a topic
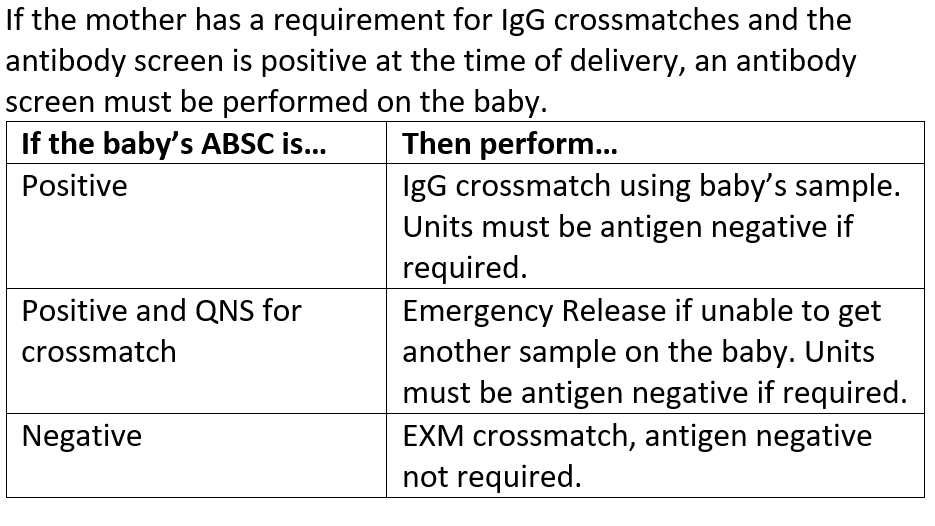
1 pointThis is an "older" policy as I am no longer with this organization, but we have a large delivery unit and a 50+ bed level I NICU. Here was our crossmatch policy for babies.1 point -
BloodBankTalk: Lan
BloodBankTalk: Lan
Malcolm Needs reacted to Darin for a post in a topic
1 pointThanks to the recent publications sent by @Malcolm Needs I knew this one! 😊1 point -
Using Meditech to track QC
1 pointWe do not have Meditech anymore, but when we did, we would create a worksheet in BBK worksheets. At first it seemed complicated but it worked out well in the end. Specially because we did not have to save paper QC for inspectors. I would just download a file to my desktop daily.1 point
-
BloodBankTalk: C(W)
BloodBankTalk: C(W)
Malcolm Needs reacted to CT1988 for a post in a topic
1 pointI just answered this question. My Score PASS1 point -
Plasma transfusions
Plasma transfusions
John C. Staley reacted to Melanie Oliveira for a post in a topic
1 pointSounds like a dilutional effect. How big is the patient and how much plasma/platelets did the patient receive during the time frame between blood draws? I have seen this with massively transfused patients and with plasma pheresis patients and with patients who have received multiple products over a few days.1 point -
Plasma transfusions
Plasma transfusions
John C. Staley reacted to bblover for a post in a topic
1 pointSeveral units of blood and plasma, in fact an MTP was called. Patient was actively bleeding.1 point -
Using Meditech to track QC
1 pointWe do BB QC in Meditech (we are in 6.x). I would not have it any other way!! I had to build it, but can pull reports, make it flag out of range QC and it is downloadable so you don't have to keep paper copies.1 point
-
A little about me
1 pointThat's really awesome and thank you for sharing! 😊 I also used to be an avid cyclist / endurance racer and have great memories of those times. Races such as the Vail 100, Brian Head 100 and 24-hour races were my "jam" during those years.1 point
-
Plasma transfusions
1 pointMy question would be - how strong was the pre-transfusion reactivity? If it was only 1-2+ in SP / and the patient isn't a "larger" person, I personally would suspect that the antibody titer was diluted to such a degree that SP did not pick it up anymore....... (but I REALLY don't like SP.....it's just too wishy-washy.....JMHO)1 point
-
Plasma transfusions
Plasma transfusions
Malcolm Needs reacted to bblover for a post in a topic
1 pointThanks for all your input1 point -
Ortho to immucor reagents
Ortho to immucor reagents
psykobillys reacted to Ensis01 for a post in a topic
1 pointQuestion to those wiser than I. Is the primary reason for these required validation comparisons to ensure we in the lab know how to follow the package inserts and so use the reagents correctly? I ask because I can't imagine any lab will be able to test the reagents to the level the manufacturer can with respect to numbers and variants (see package insert).1 point -
Ortho to immucor reagents
1 pointLike @Bet'naSBB said, there's really no easy answer. My first response is ALWAYS, check with the manufacturer to see if they have documents or guidance. Then, read the package inserts, they may have info. Then, barring anything definitive from above, you and your medical director need to review your patient population and determine how many of each type of patient you want to test. Do you have a lot of antibody patients? Do you treat cancer patients?1 point
-
transfusiom reaction analysis in QPS 7 of JCI accreditation
transfusiom reaction analysis in QPS 7 of JCI accreditation
Louella reacted to David Saikin for a post in a topic
1 pointWhen we have a suspected transfusion reaction a process of investigation is initiated: clerical checks, DAT on pre/post-tx specimens, urine for blood, ABORh on post specimen., visual exam of pre/post specs for hemolysis/icterus. If all of these are negative, the pathologist will provide an interpretation based on the reaction defined by the Nursing staff. If the investigation indicates a possible hemolytic transfusion reaction an entirely different process is initiated . . . HOWEVER, just because a reaction does not appear hemolytic it cannot be assumed that a reaction has not occurred as there are many types of reactions to blood products the majority of which are not hemolytic. It would behoove you (and your Medical Director) to research the literature to discover these and then develop the processes of investigation according to the BB standards in your area of the world.1 point -
blood bag disposal
1 pointI was the safety officer in my lab for over 10 years, and the thought of having to empty a blood bag gives me the shivers! Just too much opportunity for a splash and exposure, never mind the mess. Also, with tubing and hard plastic connectors attached to the unit, it really should be in a hard sided container. The connectors (we also call them spikes) could easily poke through a plastic bag alone.1 point
-
blood bag disposal
blood bag disposal
Louella reacted to David Saikin for a post in a topic
1 pointNursing puts post-tx blood bags in biowaste. It only comes back to lab if there is a possible reaction. We do not remove excess blood from a bag being discarded.1 point