Leaderboard
-
Points8,945Posts
-
 Points275Posts
Points275Posts -
-
Popular Content
Showing content with the highest reputation since 07/02/2024 in all areas
-
+s in Ortho panel
+s in Ortho panel
traci89 and 10 others reacted to Malcolm Needs for a post in a topic
11 pointsThe +s stands for strongly expressed. The expression of the P1 antigen varies considerably from person to person, but the reaction strength with anti-P1 is an inherited trait (i.e. the strength of the expression on the red cell surface). "I apologize for this dumb question." BBnoob69, NO QUESTION IS A DUMB QUESTION, IF YOU DO NOT KNOW THE ANSWER. If you don't know the answer, the dumb thing is to not ask the question in the first place. NEVER be afraid to ask a question on here,11 points -
anti-Jka likes to react unpredictably?
anti-Jka likes to react unpredictably?
Arno and 7 others reacted to Malcolm Needs for a post in a topic
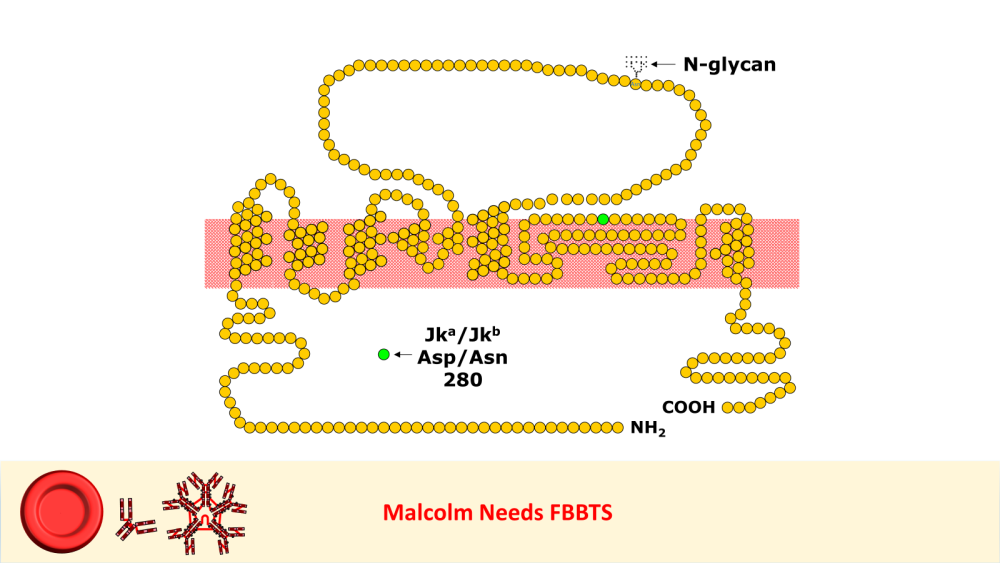
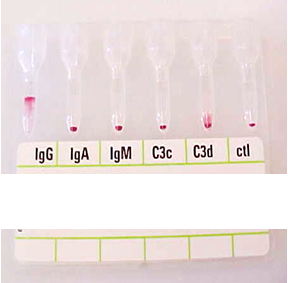
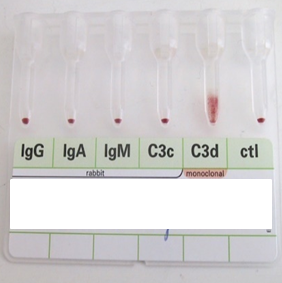
8 pointsI worked in Red Cell Immunohaematology for most of my 43 years before retirement, including two times at the International Blood Group Reference Laboratory (IBGRL), and for over a decade at one of the NHS Blood and Transplant Centres in London. During that time, I saw some pretty weird Kidd antibodies, but never came across an example of one that reacted with red cells with Jk(a) heterozygous expression, but not with Jk(a) homozygous expression. One such "weird" type (although I never saw one) was the extremely rare, dominant inhibitor type In(Jk), similar, but, of course, not identical to In(Lu). These red cells usually type as Jk(a-b-), but their true Kidd type can be ascertained by Adsorption and elution tests.. These red cells are also more resistant to haemolysis by 2M Urea than red cells with "normal" expression of the Kidd antigens, but less resistant to haemolysis by 2M Urea than true "amorphic" Jk(a-b-) red cells. There are approximately 14, 000 copies of the Kidd carrier molecule per red cell (quite a small number, when compared with some other carrier molecules, such as the D antigen). The amino acid residue that defines either the Jka or Jkb antigens is very close to the red cell membrane in the 4th extracellular loop but is largely “hidden” by the 3rd extracellular loop (steric hindrance). Both facts may contribute to the weak reactions between Kidd antibodies and Kidd antigens. Schematic of the Kidd carrier molecule (after Wester ES, Storry JR, Olsson ML. Characterization of Jk(a+weak): a new blood group phenotype associated with an altered JK*01 allele. Transfusion 2011; 15: 380-392. DOI: 10.1111/j.1537-2995.2010.02795.x. In this paper, Wester et al also describe weakened forms of both the Jka and the Jkb antigens, but in each case, the amino acid substitution is remote from position 280 of the mature protein. In addition, an individual with the Trp171Arg mutation with weak Jk(a) expression has produced an anti-Jk3 or anti-Jk3-like antibody, and so they may be “dangerous patients” (Whorley T, Vage S, Kosanka J, Lose SR, Sandquist AR, Copeland TR, Westhoff CM. JK alleles associated with altered Kidd antigen expression. Transfusion 2009; 41 (Suppl.): 48A-49A (abstract). Lastly (for now anyway!), most foetal red cells sensitised by maternal antibodies react only with anti-IgG, but I (and a colleague Grant Webb) have both noticed, but not published, occasions when such red cells also react with anti-C3d and, in one case, only anti-C3d (see genuine photographs below)..8 points -
Gel vs tube for DARA patients
Gel vs tube for DARA patients
Mabel Adams and 6 others reacted to Neil Blumberg for a post in a topic
7 pointsWe have educated our multiple myeloma specialists to send a type and screen before administering the first dose of a daratumumab (Darzalex). Our standard operating procedure is to have a panel of three cord blood cells (we have a large OB service) that is a laboratory developed test of sorts. Cord cells do not express CD38 at interfering levels. As it turns out we have made more of an issue of this than it warrants. Patients who have negative antibody screens essentially never develop new antibodies to red cells after being started on daratumumab probably because it potential inhibits B cells function. Minimal B cell function apparently yields little ability to make antibodies to red cell antigens, which are relatively weak alloantigens, especially when there is no adjuvant or inflammation in the recipient. That said, a manufacturer is making a soluble CD38 analog that will inhibit the anti-CD38 activity and make testing easier from what I've read. DTT treatment is also reasonable. But the good news is that patients on this drug do not make new antibodies. There are literature references to this, and we have probably tested about 500 patients with no new alloantibodies. Mostly non-transfused patients, obviously.7 points -
ABID Using Mixed Methodologies?
7 pointsCan you convert the tube panel cells from 3% to 0.8% and test in gel? We primarily do that to run selected cells that are not already diluted to 0/8%7 points
-
IAT & Ab ID
IAT & Ab ID
Kelly Guenthner and 6 others reacted to Malcolm Needs for a post in a topic
7 pointsColumn agglutination technology is an excellent technique, but does have a tendency to detect antibodies that react at temperatures well below 37oC, even after fairly prolonged incubation at 37oC. However, the fact that the blood group, including the "reverse grouping" is clear of atypical agglutination suggests that this may not necessarily be the case for this patient. Just to be on the safe side though, and if you can, I would either treat the plasma from the sample with rabbit erythrocyte stroma (which will adsorb out most "cold" agglutinins), treat the plasma with 0.01M dithiothreitol (which will denature the J-chains of IgM molecules, meaning that, although they can still sensitise the red cells, they are no longer able to agglutinate the red cells) or, and my personal favourite, is to pre-warm the plasma and red cells to 37oC before mixing, perform the IAT at strictly 37oC in glass tubes, wash with saline warmed to 37oC and use monospecific AHG. If any, or all, of these techniques lead to negative results, the chances are that the antibody is a clinically insignificant "cold" IgM antibody, such as an auto-anti-HI (given that the patient is group A, and the test cells are all group O).. Failing the above, send a sample to a red cell reference laboratory. I hope that helps a little bit.7 points -
New Blood Group System.
New Blood Group System.
Sherif Abd El Monem and 6 others reacted to Malcolm Needs for a post in a topic
7 pointsA research team led by NHS Blood and Transplant scientists based in Bristol, at NHSBT’s International Blood Group Reference Laboratory (IBGRL), and supported by colleagues at the University of Bristol, has discovered a new blood group, MAL. 🙌 🩸 They identified the genetic background of the previously known but mysterious AnWj blood group antigen, thus allowing identification and treatment of rare patients lacking this blood group. Louise Tilley, Senior Research Scientist, IBGRL Red Cell Reference at NHS Blood and Transplant, said: “The genetic background of AnWj has been a mystery for more than 50 years, and one which I personally have been trying to resolve for almost 20 years of my career. It represents a huge achievement, and the culmination of a long team effort, to finally establish this new blood group system and be able to offer the best care to rare, but important, patients." hashtag#NHSBT hashtag#GiveBlood hashtag#SaveLive hashtag#NHSCareers Activate to view larger image,7 points -
Transfusion requirement for patient of A3 subgroup?
Transfusion requirement for patient of A3 subgroup?
BB Gal and 6 others reacted to Mabel Adams for a post in a topic
7 pointsGiving O units (or A2) keeps our computer happier on patients with documented anti-A1. Of course, that is the prime objective these days, right? We bow to our computers!7 points -
Giving same group really that necessary
Giving same group really that necessary
John C. Staley and 5 others reacted to Neil Blumberg for a post in a topic
6 pointsPerhaps this is one rare physician who actually reads the medical literature on the subject or has thought things through. The history of this is very simple. Based upon the experience of severe or fatal hemolytic transfusion reactions to whole blood, it was discovered that when a patient's ABO type was unknown, and urgent transfusion was life saving, group O was the least likely to result in disaster. When group O red cells became available during the middle of the last century, with modest amounts of plasma left, it was decided by the then experts that this could be used for non-urgent, routine transfusions of all patients. So-called universal donor O red cells. The problem, with the 100% accuracy of hindsight, was that we had no evidence this is was good, much less optimal practice. But it was convenient. It meant blood banks didn't have to stock all 8 Rh and ABO types, so it was good for us in the transfusion service. It wasn't good for patients. Why is that? Well, there is residual incompatible plasma with anti-A and anti-B in all group O red cells that haven't been washed or thoroughly volume depleted. Well, you might ask, and all of us have assumed for decades, that a few dozen milliliters of incompatible plasma is not a big deal. The answer, now known to some extent, is that it is a big deal for some patients who are groups A, AB and probably B. This small residual plasma can on rare occasions cause severe hemolysis. It's 100% severe if it happens to you as a patient. This has been known for decades. What is new is the data that recipients of ABO mismatched red cells (Group O in general) have a higher rate of red cell alloimmunization to other red cell antigens, (Transfusion 2012 Mar;52(3):635-40. doi: 10.1111/j.1537-2995.2011.03329.x; 2025 Mar;65(3):588-603. doi: 10.1111/trf.18135. higher rates of febrile and allergic reactions, (Transfusion 2012 Mar;52(3):635-40.doi: 10.1111/j.1537-2995.2011.03329.x.) higher rates of HLA alloimmunization, and perhaps overall higher rates of mortality (Transfusion. 2016 Mar;56(3):550-7.doi: 10.1111/trf.13376). So, if you are a recipient, you want ABO identical transfusions, or compatible red cells that have had all or almost all of the plasma removed, as by washing, for example.6 points -
AABB Individual Membership
AABB Individual Membership
Kelly Guenthner and 5 others reacted to jshepherd for a post in a topic
6 pointsMy facility is not AABB accredited, just Joint Commission and FDA, and I have an individual membership. I am the supervisor, and we are a large level 1 metropolitan trauma hospital. I have gained so much from my membership. Everything Cliff mentioned about resources is true, and I can't tell you how many times we've taken advantage of the discount on books for my pathologists (none of whom are transfusion medicine specialists). AABB membership also opened up all the subcommittees and sections, and I now sit on 9 subsections and lead one of them. I am also a mentor in the program Cliff mentioned above. I would say it's worth it for at least one year, so you can try it out and see what you get from it. Pro tip: if you love it, they do offer a 3 year membership option that knocks some of the cost down.6 points -
Whole Blood
Whole Blood
Mabel Adams and 5 others reacted to Neil Blumberg for a post in a topic
6 pointsSwitch back to the patient's own ABO type as soon as possible is my advice. For everything. RBC, platelets, cryo, plasma. Worrying about the anti-A and anti-B in low titer whole blood is relevant, but so is the smaller amount of incompatible plasma in group O red cells, which are not low titer. There are rare reports of severe hemolytic reactions to group O red cells in non-O patients. Furthermore, the patient is continually making their own group A, B or AB red cells, so hesitancy about transfusing their own ABO type is not helping things get better. By giving additional group O products we are making the problem worse, not adding safety in any way. Furthermore, the non-O patient's endothelial cells, platelets, von Willebrand factor, hepatocytes, etc. are all incompatible with the transfused group O plasma, and their function is impaired when modeled in vitro, and leads to increased bleeding. Thus there is no benefit whatever in giving group O red cells (or whole blood for that matter) to non-O patients once the hemorrhagic problem is largely under control. And there is likely added risk. It is only adding harm and reducing the inventory of group O blood for group O recipients. A total mistake of the last few decades in my opinion. Giving group O plasma containing products to non-Os is only reasonable when you don't know the patient's blood group, or don't have their blood group in stock, or it's an emergency with no time for giving type specific. No one ever went broke overestimating the importance of the ABO blood group in transfusion. See attached for the literature references. ABO trauma commentary Frontiers bioengineering.pdf Reconsider ABO compatible:universal donor.pdf ABO ARC MAC copy.ppt6 points -
Pipette usage for elution
Pipette usage for elution
psykobillys and 5 others reacted to Bet'naSBB for a post in a topic
6 pointsUsing a new pipette each time is how I keep track of how many times I've washed!..........4 washes with Working Wash? - Lay out 4 pipettes!........AND close my drawer so I won't - out of habit - reach in and get one! LOL! (I'm and "older tech" too! ) but as to your question........I don't know the true answer......... we always use a new one. But, if it does not state specifically in the IFU and their eluates / LW's give valid results.........I'd say it doesn't matter..........???6 points -
Transfusion requirement for patient of A3 subgroup?
Transfusion requirement for patient of A3 subgroup?
Mabel Adams and 5 others reacted to John C. Staley for a post in a topic
6 pointsI believe the old saying is, "If the computer ain't happy, ain't nobody happy!!"6 points -
Source of advice on transfusing patients with mismatched stem cell/bone marrow transplants?
Source of advice on transfusing patients with mismatched stem cell/bone marrow transplants?
Arno and 4 others reacted to Malcolm Needs for a post in a topic
5 pointsSorry Neil, but I have to point out that this is not completely accurate. Any red cell antigens that are adsorbed onto the red cell surface, rather than being an integral part of the red cell membrane remain the type of the patient, rather than the donor. This is true of the Lewis phenotype (for instance, if the recipient was Le[a+b-], and the donor was Le[a-b+], after the transplant, the red cells will group as Le[a+b-], and not as Le[a-b+]}. This is also true of antigens within the Chido/Rodgers Blood Group System, and certain others. If the recipient is a Secretor, they will continue to secrete ABO substance of the original ABO type, which, of course, will also be adsorbed onto the red cell surface (as well as being in the plasma, leading to the phenomenon of "accommodation", and this is why most recipients stay with a reverse group of "AB" after an ABO mis-matched stem cell/bone marrow transplant. SORRY TO BE A PEDANT, PARTICULARLY AS I AGREE WITH EVERYTHING ELSE YOU HAVE WRITTEN!5 points -
Eluate in the Laboratory Diagnosis of Autoimmune Hemolytic Anemia
Eluate in the Laboratory Diagnosis of Autoimmune Hemolytic Anemia
Neil Blumberg and 4 others reacted to Malcolm Needs for a post in a topic
5 pointsMany, many years ago now, when I was working at the old Westminster Hospital in London as a quite junior member of the Blood Transfusion staff, I spent quite a few hours working on a sample from a patient with a positive DAT, trying to determine the specificity, in order to see whether the antibody was an allo- or an auto-antibody. This included the use of several very rare red cells that I had frozen down and also examining the eluate. After many happy hours, I had got precisely nowhere, and so sent a sample to my former colleges at the International Blood Group Reference Laboratory. I received a somewhat "spicy" report from my heroine Joyce Poole, who explained to me, in words of one syllable, that I had rather been wasting my time, rare cell collection and the laboratory's money as, in almost all cases, the specificity would be found to be an auto-anti-Rh17 or auto-anti-Rh18!!!!! Since then, I have reverted to doing as little as possible on such samples and, when I retired 43 years later, and, as far as I know, none of the patients ever died as a result. There was a close one once, when I was working on a sample on a Saturday on-call in the Red Cell Reference Laboratory at NHSBT-Tooting Centre. I was working on a sample that was overtly a case of wAIHA. After several allo-adsorptions, I was finally able to provide "suitable blood". It was only at the last minute that the computers came back on after an unplanned downtime, and it appeared that the patient was known, from many years previously as having an allo-anti-Vel!!!!!!! As you can imagine, I did several more tests before I released the blood (on Pathologist's orders), as there was absolutely no evidence of an anti-Vel (auto- or allo-) in the present sample - but it did give me a bit of a turn!!!!!!!!!!!! I would recommend a thorough reading of Petz LD, Garratty G. Immune Hemolytic Anemias. 2nd edition, 2004, Churchill-Livingstone. ISBN 978-0-443-08559-8. Other than that and, possibly, looking at the patient's sample at a more molecular level (as noelrbrown suggests above), I really wouldn't do much else, except that I would put a little note with the blood to be transfused to remind the doctors and nurses on the ward to be vigilant with their patient observations. ALL OF THE ABOVE HAVING BEEN SAID, I AM NOT, AND NEVER HAVE BEEN, MEDICALLY QUALIFIED!!!!!!5 points -
Antibody Work-up
Antibody Work-up
Ensis01 and 4 others reacted to exlimey for a post in a topic
5 pointsThis a classic case of Sensitivity vs Specificity. Clinical assays (including those in the transfusion medicine arena) are often designed to be as sensitive as possible, especially those intended to be frontline screens. Nobody wants to miss anything. However, the downside of this approach is that sometimes specificity suffers - more positives are obtained than desired. Solid Phase, Gel and PEG assays are super-sensitive, but may detect "nuisance" antibodies like weaker autoantibodies (warm and cold), or things like anti-P1, anti-Leb, or "HTLA-like" antibodies. Less sensitive assays that employ no enhancement (saline) or thing like LISS are sometimes used strategically to avoid the nuisances. Many institutions have policies that allow the deliberate down-regulation of sensitivity to ameliorate such problems. For example, performing crossmatches in LISS rather than Gel. Certainly, there's a risk that something might be missed using a less sensitive assay, but I think it's important to realize that in years past, the use of those less sensitive assays didn't leave a trail of bodies. In patients with long-term autoantibodies, it's not unusual for the autologous cells (DAT/autocontrol) to react weaker than Screening Cells or panel cells. The appears to be some kind of weakening of native antigens to which the autoantibody is directed. Sometimes, that may even mean the DAT is negative, but still yields a reactive eluate .5 points -
Transfusing high Hgb because "patient still in shock"?
Transfusing high Hgb because "patient still in shock"?
Yanxia and 4 others reacted to Neil Blumberg for a post in a topic
5 pointsHemoglobin and hematocrit re-equilibrate over minutes to an hour. Usually minutes. Not five hours. That's not compatible with what we know. Transfusing a patient in this setting is more likely to cause inflammation, thrombosis, congestive heart failure, etc., than help, although it is understandable that the surgeon is trying to "do something" for a patient who is not responding to treatment. The hemoglobin may or may not be precise, but it tells you that the circulating red cell mass isn't likely the problem. If the patient is in shock and not bleeding, the problem is almost certainly not fixable by transfusion of red cells is my thought. But desperation leads people to try stuff that is unlikely to help and may, in some cases, harm. Likely cause(s) of the shock in such patients is cardiac dysfunction, sepsis or something else not easily fixable. Not due to anemia/lack of red cell mass obviously.5 points -
JCAHO - Issuing blood products
JCAHO - Issuing blood products
Ally and 4 others reacted to Mabel Adams for a post in a topic
5 pointsUnder OSHA, tested donor blood is not considered biohazardous. For heaven's sake, we infuse it into patients! It would take me some time to find the regulation, but it is in the OSHA regs.5 points -
Plasma transfusions
Plasma transfusions
Kelly Guenthner and 3 others reacted to Neil Blumberg for a post in a topic
4 pointsAlso, were any of the transfused units antigen positive? This is the quickest way to get a negative indirect antiglobulin test ;).4 points -
Need Advice
Need Advice
Ensis01 and 3 others reacted to jojo808 for a post in a topic
4 pointsMy greatest apology for leaving you all hanging. We've been so incredibly busy and short-staffed that I could not even think about anything else but trying to finish up my daily duties. Anyway, seems that the patient also had an impella device that had to be "adjusted" and I believe that corrected the hemolysis. I'm only reading the responses today (2 weeks later)☹️so hats off to you all who thought mechanical causes. I would have not thought that the device would be that far-off to cause the gross hemolysis we saw. We do see slight hemolysis with impella devices but not like this one. I guess never say never. Thank you all for your responses.4 points -
Proper storage space for pRBCs while in refrigerator
Proper storage space for pRBCs while in refrigerator
Kelly Guenthner and 3 others reacted to BBMT for a post in a topic
4 pointsI am retired now and will never forget the sound they make when they are dropped on the floor.4 points -
Electronic crossmatch in ABO discrepancies?
Electronic crossmatch in ABO discrepancies?
applejw and 3 others reacted to jshepherd for a post in a topic
4 pointsThe FDA Guidance on Computer Crossmatching calls out that patients with an ABO typing discrepancy should not be allowed to qualify for computer crossmatches. " If ABO typing discrepancies exist, you should not rely on a computer crossmatch. This is particularly important if there is mixed field red cell reactivity, missing serum reactivity, or apparent change in blood type following hematopoietic stem cell transplantation. Under those circumstances, your procedures should provide for compatibility testing using serologic crossmatch techniques." I wrote our policy to include any non-straightforward ABO types for any reason will be required to get an IS or IAT crossmatch.4 points -
Source of advice on transfusing patients with mismatched stem cell/bone marrow transplants?
4 pointsThis may be a little outdated, it's from a prior facility. We did a tremendous amount of transplant infusion, and this evolved over the decades I was there. Management of Hematopoietic Progenitor Cell Transplant Recipients.docx4 points
-
Unusual A type
Unusual A type
Mabel Adams and 3 others reacted to Malcolm Needs for a post in a topic
4 pointsI was thinking of antigens such as M, N, S, s, Fya, Fyb, Jka and Jkb. However, they would only tell you if the patient is likely to be a chimera. As far as what to give the patient, you are right in saying that these results would not help too much, if at all. From what you have described, group A, D Positive cross-match compatible units should be fine.4 points -
AABB Individual Membership
AABB Individual Membership
Ally and 3 others reacted to John C. Staley for a post in a topic
4 pointsI never regretted my individual AABB membership. They can be an excellent resource, especially in your new position. Having said that, I imagine things may have changed since my retirement. I would suggest getting a membership, if you are not seeing the cost: benefit ratio in your favor you can always cancel or just not renew.4 points -
Eluate in the Laboratory Diagnosis of Autoimmune Hemolytic Anemia
Eluate in the Laboratory Diagnosis of Autoimmune Hemolytic Anemia
jtemple and 3 others reacted to snance for a post in a topic
4 pointsAgree with Dr Blumberg. In an abstract from way back, 1996, Ann Church studied the value of an eluate, reference and abstract below (apologies for quality of print). A Church, S Nance, D Kavitsky. Assessment of Elution Studies in Cases with 37OC Reactive Serum Autoantibodies (SA). Transfusion 1996; 36:161S (Suppl)4 points -
Eluate in the Laboratory Diagnosis of Autoimmune Hemolytic Anemia
Eluate in the Laboratory Diagnosis of Autoimmune Hemolytic Anemia
mpmiola and 3 others reacted to Neil Blumberg for a post in a topic
4 pointsAgree that in “not recently transfused”patients, eluates are of no clinical use unless you like seeing panagglutinins.😜 Negative eluates occur in some drug dependent examples but the clinical information is paramount in such situations in any case.4 points -
Non specific antibody
Non specific antibody
Prince Domson and 3 others reacted to Malcolm Needs for a post in a topic
4 pointsIt is most unlikely to be anti-f, as this specificity will not react with either R1R1 or R2R2 red cells (I presume that you are using both antibody screening cells and antibody identification panel cells that have these phenotypes represented). Anti-f reacts with red cells where the c and e antigens are the result of an RHCE*ce haplotype where, for want of a better way of putting it, the c and e antigens are the result of the RHc and RHe genes being in the cis position - NOTE THAT THIS TERMINOLOGY IS (KNOWINGLY) WRONG! Like all Rh antibodies, anti-f reacts most strongly with its cognate antigen on red cells that have been treated with a proteolytic enzyme (such as papain or ficin), but will very often react with untreated red cells by IAT.4 points -
Cold Stored Platelets -interpreting FDA guidance
Cold Stored Platelets -interpreting FDA guidance
jtemple and 3 others reacted to Neil Blumberg for a post in a topic
4 pointsI think you do what is practical in your setting. There is no evidence one way or the other for prophylactic use of cold vs. room temp platelets in terms of actual bleeding prevention. Platelet count increments were the metric used, and we now know that platelet count is a relatively minor contributor to bleeding at counts much above 5-10,000/µl. So we're all operating based upon "expert" (often wrong) opinion, not actual data. In general, at platelet counts above 10,000/µl there is virtually no evidence of benefit for platelet transfusion and plenty of evidence of harm (particularly if not ABO identical). So the best clinical decision is often to postpone or eschew transfusion in my view. The long standing view that transfusion is almost always better than no transfusion is tragically wrong.4 points -
Antibody Work-up
Antibody Work-up
Ensis01 and 3 others reacted to Malcolm Needs for a post in a topic
4 pointsI tend to think that there are a number of factors affecting the reaction, such as changes to the pH, rather than just dilution, that would change the equilibrium constant within the Law of Mass Action that governs antibody/antigen reactions, but pH is only one of them.4 points -
Transfusing high Hgb because "patient still in shock"?
Transfusing high Hgb because "patient still in shock"?
BB Gal and 3 others reacted to Neil Blumberg for a post in a topic
4 pointsJust for the record, I am not aware of any data, nor can I conceive of a mechanism by which red cell transfusion would correct base excess. This patient apparently had an extensive and severe infection, so vasodilation due to septic shock seems a real possibility. Transfusing a patient to a hemoglobin of 14.6 is not something I've ever heard experts in anesthesiology and intensive care medicine advocate, and transfusion to this level would be expected to increase the risk of thrombosis greatly. And just for completeness, the diagnosis of septic shock in a patient with a recent serious infection and also likely receiving broad spectrum antibiotics does not require the presence of positive cultures for diagnosis. Hard to grow bugs in vitro when there are high concentrations of anti-microbials. This is why DNA tests are probably a better tool to diagnose infection in such patients, if available. Does not require growth, just the presence of bacterial nucleic acids.4 points -
Blood used organ donation services
Blood used organ donation services
Arno and 3 others reacted to Neil Blumberg for a post in a topic
4 pointsThe standards probably don't apply to post-mortem transfusions. I cannot imagine why an organ harvest surgery would require transfusion at all, but I'm not a surgeon. I've had requests to transfuse platelets and plasma to organ donor patients, which we've uniformly denied. I'd need some explanation of why transfusion, which is pro-inflammatory, immunomodulatory and pro-thrombotic, would benefit the potential organ recipient. There is so much misinformation, partially promulgated by the transfusion medicine community, of the "benefits" of transfusion, that it is sometimes difficult to explain to clinicians why transfusion is a bad idea in many situations.4 points -
ABO Retypes
ABO Retypes
jnadeau and 3 others reacted to Neil Blumberg for a post in a topic
4 pointsThe absence of a second sample leading to transfusing group O to everyone without a second sample is an example of why this practice is likely doing more harm than good in my view. Group O red cells are acceptable for all in an emergency, but they are potentially harmful to non-O recipients due to the presence of 20-30 ml, give or take, of incompatible plasma for A, B and AB patients. There are case reports of severe hemolytic reactions in this setting. Low level hemolysis that is not clinically evidence is present in some recipients and is associated with thrombosis, infection and organ injury in animal models. In experiments of nature such as sickle cell anemia/paroxysmal nocturnal hemoglinuria below visually apparent levels of free hemoglobin cause severe complications. Thus, contrary to long accepted practice, Group O is NOT universal donor, except in emergencies when the recipient's type is not known, or the patient's own ABO type red cells are unavailable and there is life threatening bleeding or anemia. The routine use of group O red cells for everyone in routine transfusion is an unfortunate practice that has arisen due to convenience and erroneous assumptions of equivalent safety. Not a good practice for patient safety.4 points -
Selecting units for patient with anti-Lea
Selecting units for patient with anti-Lea
John C. Staley and 3 others reacted to Neil Blumberg for a post in a topic
4 pointsLewis antibodies do not cause HDFN and do not need titration. We do not Lewis phenotype transfusions to patients with anti-Lewis antibodies, but do a manual antiglobulin crossmatch to find units that do not react. I'm sure there are rare patients whose antibodies can cause removal of Lewis positive red cells at an accelerated rate, but this is not something that needs to be considered unless the patient shows signs of hemolysis or rapid red cell removal. Never come across this in 50 years of practice :). But never say never in medicine.4 points -
anti-Jka likes to react unpredictably?
anti-Jka likes to react unpredictably?
monopolova and 3 others reacted to psykobillys for a post in a topic
4 pointsI've seen it at least 2, maybe 3 times where reactions were consistent (ie repeatable) but didn't behave as expected - first of all, generally weak reactions, nothing stronger than a strong 1+ in gel, but reacted with some (not all) homozygous Jka cells and some (not all) cells that were heterozygous. each time, we threw more cells at it to prove/disprove the anti-Jka conclusion and each time the only consistency was that all of the reactive cells had the Jka antigen. could it have been some other antibody for which our panel cells didn't note the corresponding antigen? sure. but i wouldn't ignore the consistent presence of the Jka antigen on the reactive cells...4 points -
Gel vs tube for DARA patients
Gel vs tube for DARA patients
traci89 and 2 others reacted to jtemple for a post in a topic
3 pointsWhat? All this time I have been using the wrong stuff! Ha! SPELL CHECK IS NOT YOUR FRIEND! 🤣🤣🤣🤣3 points -
Plasma transfusions
Plasma transfusions
AuntiS and 2 others reacted to Malcolm Needs for a post in a topic
3 pointsI agree Darin, it is almost certainly a dilutional effect, BUT, it could also be the effect of a soluble antigen (obviously not within the Duffy Blood Group System). If the antibody had a specificity within, for example, the Lewis Blood Group System, or the Chido/Rodgers Blood Group System, the antigen in the plasma could well adsorb out the circulating antibody. That having been said, this explanation is FAR less likely than your suggestion of the dilutional effect.3 points -
Welcome Darin
Welcome Darin
Malcolm Needs and 2 others reacted to Darin for a post in a topic
3 pointsThank you so much! I've been perusing the site for a few years and thought, hey, why don't I sign up! LOL I'm the BB Lead at a smaller hospital in AZ and have 30 years experience. Looking forward to learning more here and, hopefully, even providing a few answers along the way.3 points -
Suspected Anti-D + Anti-C vs. Anti-G: Separation/Differentiating Difficulty — Has Anyone Seen Similar Results? Case Comparisons? Thoughts...
Happy Friday everyone! First off, Thank you Malcolm 😊 for that excellent PPT lecture! 👌 With your permission, I'd love to share it with my students; it's exactly the kind of content that helps bring these complex concepts to life (and mildly melt brains in the best way possible). Speaking of melted brains… let's talk Anti-G differentiation, shall we? While I agree that differentiation isn’t necessary for transfusion purposes because in cases where anti-G is suspected, the recommendation is the same (D and C antigen-negative PRBCs); in the US Reference Lab world, it's a whole different dance. Differentiating anti-D + anti-C from anti-G is essential in alloimmunized patients with anti-D + anti-C for Rh Immune Globulin (RhIg) prophylaxis administration indication. Medical teams want to know if a patient is a candidate for RhIG, and if it's still indicated, especially to avoid future medical and legal complications. And so begins the beautiful (read: painstaking) double adsorption and elution process. 😫 The presence/development of a real anti-D indicates the patient does not require RhIg administration, whereas the presence of anti-G indicates the need for RhIg prophylaxis. That way, RhIG is avoided in patients with a real anti-D (although clinical correlation is recommended to guide every decision). In this case, we saw what looked/reacted like anti-D and anti-C (1). Adsorption with R2R2 cells showed anti-C in the adsorbed serum (2) and anti-D and/or G in the adsorbing R2R2 cell eluate (3). Usually, we would stop and call anti-C and anti-D, if we have no reactions on C Ag Pos cells, but since suspected anti-G was in the mix, we routinely differentiate/separate it. The anti-D was confirmed in (4) by adsorbing the eluate containing the suspected (anti-D + anti-G) with an r'r unit. So far, so good. However, when we tried to separate the anti-G by elution of the adsorbing r′r cells, expecting a reaction on D and C antigen-positive cells (5), we got a negative result (Cue dramatic music). I know, I know...at this point you’re probably wondering if we’re still in the Blood Bank or if we’ve accidentally wandered into theoretical physics. Trust me, my brain also wobbles when explaining G differentiation. It's the kind of thing that makes you rethink your career choices... for about 5 minutes... and then roll up your sleeves and start prepping another adsorption. 😅 A couple of housekeeping: We ficin treat our adsorbing cells, and sometimes use PEG in adsorption for efficiency. After briefly considering a career in something less chaotic, we retraced our steps. No PEG this time, 60-minute incubation, followed up with a DAT after each adsorption to check for antibody coating... and voilà: finally got the positive reaction we were looking for. The patient has anti-D, anti-C, and anti-G. I can only think of a possible weak DAT strength pending elution, or PEG interference when used in adsorption. As always, this is Blood Bank, we know a million things can go wrong, and often do. But in the end, science (and a lot of perseverance) wins. Thanks again to everyone for your input, patience, your brains, and your sense of humor. And again, Malcolm, thanks for your lecture, teaching, dedication and inspiration! I wish everyone a calm weekend. May your panels be clear, your DATs negative, and your eluates informative. 😉3 points
-
Suspected Anti-D + Anti-C vs. Anti-G: Separation/Differentiating Difficulty — Has Anyone Seen Similar Results? Case Comparisons? Thoughts...
Suspected Anti-D + Anti-C vs. Anti-G: Separation/Differentiating Difficulty — Has Anyone Seen Similar Results? Case Comparisons? Thoughts...
DLabGirl and 2 others reacted to Malcolm Needs for a post in a topic
3 pointsIn the UK, we would test serum/plasma samples from pregnant patients to see if there was an anti-C + Anti-G, or an anti-G on its own, but if the tests showed an anti-D+C, we didn't go any further to see if there was an anti-G there as well. I mean, what for? What difference does it make? I attach a PowerPoint lecture on the subject I wrote some years ago, but I think it is still pertinent. The G Antigen and Anti G.pptx3 points -
Giving same group really that necessary
Giving same group really that necessary
Arno and 2 others reacted to Malcolm Needs for a post in a topic
3 pointsNeil Blumberg, I'll leave this one to you!3 points -
FDA requirements for 2 people to issue blood products for transfusion.
2 people at sign out or issue is not required.3 points
-
FDA requirements for 2 people to issue blood products for transfusion.
FDA requirements for 2 people to issue blood products for transfusion.
applejw and 2 others reacted to Neil Blumberg for a post in a topic
3 pointsAs far as I know there is no FDA requirement for two people to issue blood. Obviously some hospitals have only one person working night shift in the lab, so that isn't happening realistically. There is a traditional requirement for two people to identify the recipient and the transfused product, but this is only if positive patient identification is not used these days.3 points -
Patient with anti-D, anti-C is negative with other reagent cells but incompatible with most units.
Patient with anti-D, anti-C is negative with other reagent cells but incompatible with most units.
Mabel Adams and 2 others reacted to Yanxia for a post in a topic
3 pointsThanks Mabel for your explanation. I think we can exclude the protein factor. 1.In my work, I have noticed that the reagent cells express less H antigen than the donor cells do. Our screen and panel cells have 3-month shelf life, but our donor cells only have about 35 days. Even though we do our best to preserve the antigens on panel cells, there are still some losses. Of course, there are other antigen loss other than H. 2.I have read lewis antibodies may react with A type, sorry I can't recall it exactly, I will check it out after this work shift when I get home. If there is an anti-A Lewis antobody, it will react stronger with donor cells. As to the incompatible O donors, my bold guess is they express more Lewis antigen than reagent cells. Sorry again for my imagination.3 points -
ABID Using Mixed Methodologies?
ABID Using Mixed Methodologies?
kab1 and 2 others reacted to Malcolm Needs for a post in a topic
3 pointsWe frequently did this in the Reference Laboratory where I was the Manager (but you have to include both a positive and a negative from the "gel" technique into the "tube" technique - or vice versa, to ensure that the "sensitivity" of both techniques, while not being necessarily identical, are reasonably close).3 points -
Whole Blood
Whole Blood
Ensis01 and 2 others reacted to Neil Blumberg for a post in a topic
3 pointsIf the donor antibody screen is negative then the unit does not need to be labeled. Otherwise it does.3 points -
Antibody Work-up
Antibody Work-up
SbbPerson and 2 others reacted to Yanxia for a post in a topic
3 pointsI guess the solid phase is more sensitive to detect the auto in this patient. I will use the same method as detecting the antobodies to do crossmatch. Of course I will do saline and AHG crossmatch as well. Just my humble opinion.3 points -
Blood used organ donation services
Blood used organ donation services
Mabel Adams and 2 others reacted to sgoertzen for a post in a topic
3 pointsAt my institution. the Donor Network is now asking for 4-6 units of RBCs for organ perfusion for their machine after the organ has been harvested (similar to ECMO for the organ). Those RBC units will not ever touch the organ donor patient. Our policy is to always issue them the oldest O Pos units (uncrossmatched) we have on our shelf. They will rinse all of this banked blood out of the organ before transplanting it into the recipient, and it is added to the perfusate solution to provide oxygenation to the organ during transport from the donor hospital to the recipient hospital. AABB offered at least 1 very informative session at their annual meeting on this last year in Nashville, and I'm guessing that they will offer more in Houston this year (or perhaps an eCast session or articles in AABB News or Transfusion) since this practice is becoming more and more widespread. The unique nature of the process is proving to be a challenge for hospital transfusion services as far as who places the order, what testing is needed (if any), tracking for final disposition, what kind of records need to be kept because they are not being "transfused", billing of the products, etc..3 points -
ABO Retypes
ABO Retypes
jnadeau and 2 others reacted to Bet'naSBB for a post in a topic
3 pointsWe have A LOT of outpatients. Using our Outpatient Dialysis patients as an example - If we do not have a second type on them, we will give O units and do an ISXM on their first presentation. Upon subsequent presentation for transfusion, another sample would be received at which time we will do a second ABO/Rh and barring any discrepancies - will then EXM units provided the screen is negative.3 points -
ABO Retypes
ABO Retypes
jnadeau and 2 others reacted to Neil Blumberg for a post in a topic
3 pointsAgree that if not for transfusion purposes, ABO types do not need repeating. We don't do confirmations for potassiums or troponins either.3 points -
anti-Jka likes to react unpredictably?
anti-Jka likes to react unpredictably?
monopolova and 2 others reacted to Malcolm Needs for a post in a topic
3 pointsI should have also said that there is an excellent Blood Group Review available, viz Hamilton JR. An update to Kidd blood group system. Immunohematology 2024; 40: 28-33. DOI: 10.2478/immunohematology-2024-005.3 points