sgoertzen
Members
-
Joined
-
Last visited
-
Country
United States
-
Wellsky Transfusion (HCLL) Emergency Issue Module
WellSky has made some dramatic changes in their latest version to their Emergency Issue module. What version are you currently on?
-
-
Method Validation for Immucor Echo
Immucor (Werfen) supplies you with all the validation documents that you need in a nice binder. You just need to fill out all of the forms and attach the print-outs.
-
-
-
-
Correlation Testing in Blood Bank
Here is the form that we use. You need to have something written in policy that accounts for the expected variability of reactions when comparing different methods. We have multiple methods for ABO/Rh, Antibody Screen, Antigen Typing, Antibody ID, and AHG Crossmatch, so we have to do method comparison on all of these. TQ-0530F03 Method Comparison__blank_copy_id_10835032.pdf
-
"Keep Ahead" Orders
We used to have a "Keep Blood on Hand" (i.e. Keep Ahead) with our old computer system, but we discontinued that when we moved to Epic. I think it was a hold-over from "way back when" before electronic crossmatching. It was time for it to go!
-
-
Blood used organ donation services
At my institution. the Donor Network is now asking for 4-6 units of RBCs for organ perfusion for their machine after the organ has been harvested (similar to ECMO for the organ). Those RBC units will not ever touch the organ donor patient. Our policy is to always issue them the oldest O Pos units (uncrossmatched) we have on our shelf. They will rinse all of this banked blood out of the organ before transplanting it into the recipient, and it is added to the perfusate solution to provide oxygenation to the organ during transport from the donor hospital to the recipient hospital. AABB offered at least 1 very informative session at their annual meeting on this last year in Nashville, and I'm guessing that they will offer more in Houston this year (or perhaps an eCast session or articles in AABB News or Transfusion) since this practice is becoming more and more widespread. The unique nature of the process is proving to be a challenge for hospital transfusion services as far as who places the order, what testing is needed (if any), tracking for final disposition, what kind of records need to be kept because they are not being "transfused", billing of the products, etc..
-
-
-
Trauma Alert Procedures?
We ended up getting an undercounter Helmer fridge to hold 2 O Neg RBC units. This fridge is locked and hooked up to the Pyxis in the Trauma Bay in the ED. They must use the Pyxis to open the fridge and access those RBC units which triggers an alert to our Trauma pager up in the blood bank. This works well and has saved us so much time and resources because we no longer have to pack up units in a cooler and run them down to the ED on all level 1 traumas. It's well worth the cost of the fridge.
-
AABB 1.4 and 1.4.1 Risk Assessment
I would recommend adding "Risk Assessment" and "Risk Mitigation" as new sections in your Variance tracking system that must be completed by the reviewer for each variance documented. You should also add it to any of your internal audit report forms, equipment corrective action forms, and proficiency testing result review reports.
-
MaxQ MTP Coolers 3.0
I would be happy to share our validation plan for this cooler. Please send me a private message with your email.
-
-
Cold auto antibodies
This is also our policy. If the cold antibody is so strong (3+ to 4+) that we must use pre-warm tube testing in order to do the workup, we advise them to use a blood warmer regardless of the location where the transfusion is being given.
-
-
Psoralen Treated Platelet in Wellsky
We have no problem with this. I will connect you with our WellSky expert who built the system to work beautifully for us. Her name is Jill and her email is JHShaw@valleychildrens.org Please reach out to her and she can walk you through exactly how to do this. I will alert her to be expecting an email from you!
-
Digitizing Antibody Workup Files
We have WellSky and Epic and we scan everything and attach it to the patient in WellSky so it becomes a part of the patient's viewable blood bank testing history.
-
New PPID Rule Scripts
If you are using Epic, I can share the contact information with you to the people at my facility that set this up for us. Our Epic/Beaker system checks for appropriate PPID scanning at the time the specimen is received into the lab. If it fails, the sample is rejected unless it has the date/time and 2 sets of employee IDs written on the tube, but if the patient has no ABO/Rh history on file, then a 2nd specimen is also required.
-
-
Standard method for isoheme titers?
I've attached copies of our procedure and our worksheet. Our Heme/Onc docs also order them on our patients post-transplant, and we occasionally get them ordered on kids where they suspect some sort of immune deficiency disease. TO-310 Isohemagglutinin Workup - Test and Titer__uncontrolled_copy (2).pdf TO-310F01 Isohemagglutinin Test and Titer Worksheet__blank_copy_id_8428444.pdf
-
-
PEDIATRIC MASSIVE TRANSFUSION PROTOCOL
I've attached our MTP procedure and worksheet. We are a children's hospital with a level 2 pediatric trauma center.Massive Transfusion Protocol - MTP.pdfMassive Transfusion Protocol - MTP Worksheet.docx.pdf
-
Beaker Result Entry
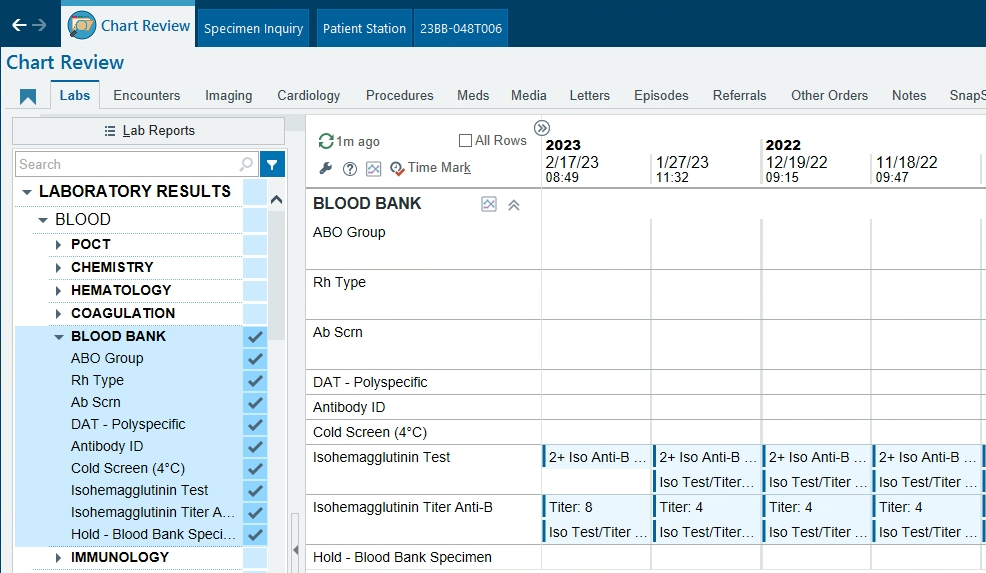
I work at a children's hospital and we use WellSky and Epic/Beaker. We built an orderable test called "Isohemagglutinin Test and Titer" specifically for reporting out the presence and strength of Anti-A and Anti-B. This test is not affiliated in any way with the regular patient Blood Type test. It's mainly ordered at my facility to monitor and follow patients with immune deficiencies or who have had an ABO mis-matched bone marrow or stem cell transplant, but it sounds like it would also meet your needs with heart transplants. I've attached our procedure and worksheet. This is how the results display in Epic. If you're interested in building something like this, I can put you in contact with our WellSky & Beaker IT gurus who built this for us. My contact is sgoertzen@valleychildrens.org TO-310 Isohemagglutinin Workup - Test and Titer__uncontrolled_copy (1).pdf TO-310F01 Isohemagglutinin Test and Titer Worksheet__blank_copy_id_7905995.pdf