Content Type
Store
Profiles
Forums
Blogs
Events
Frequently Asked Questions
Gallery
Downloads
Glossary
Links Directory
Questions
Jobs
Vendors
Posts posted by jayinsat
-
-
2 hours ago, exlimey said:
What is the ABO group of the patient and of the crossmatched units ?
My initial thought is check for ABO compatibility, which is what exlimey is suggesting. How was the Jka antigen testing performed? Were controls run and did they work? Also, perform a DAT on the units. If they are DAT positive, your crossmatches are invalid. I doubt that would be the issue though. 4 donors all having positive DAT's? If you had a clear anti-Jka identified, and all other antigen groups ruled out homozygously, there is no reason the units should be XM incompatible if they are ABO matched.
-
I would treat this like any other lookback or non conforming product notification from my supplier. I would generate a risk management occurrence report and submit it to our medical director. Our medical director and the patient physician would figure this all out.
-
To add to the this thought, the low titer O whole blood currently being used in many trauma situations is not leuckoreduced or irradiated. Seems to me that it is time to rethink the need to irradiate in trauma situations.
-
20 hours ago, pbaker said:
How does everyone ensure that red cell units stay within temp while receiving them into the BB inventory? Do you designate a time allowed from removal from the shipping box to placement in the refrigerator? Do you take temps somehow? Do you document anything anywhere?
Is there a new checklist item that is asking about this? Otherwise, I see no need to record this. We do not record the temp when it is out for antigen typing or any other tests. Why do it here?
-
On 12/4/2021 at 3:26 PM, Danielle said:
Side note, I totally love the snowflakes and Christmas lights going on! Tis the season!
Thanks!
Danielle,
I love the lights. When i'm feeling stressed, I come here and break em. If you haven't noticed, move over them with your mouse and they break with the most refreshing sound of glass breakage.
I hope you all don't think less of me now...
-
On 12/3/2021 at 9:15 PM, Danielle said:
Hi all,
Our Fetal Screen package insert says that a positive maternal DAT can cause a falsely positive Fetal Screen. Does a positive Fetal DAT have any effect on the validity of the Fetal Screen? The package insert does not mention any.
Thanks in advance!
I would consider this a non-issue as you would need to quantify any positives with a KB stain or flow cytometry anyway.
-
We moved rhogam to pharmacy more than 10 years ago for all 7 of our hospitals in our system. It has been great! It poses no more issues than any other transfusion or lab related process that is under other-than-lab control (POC, transfusion administration, etc). We get a report printed from pharmacy (autogenerated) every morning that inform us when patients received rhogam, fludara, or IVIG. We print a report daily in blood bank that shows all rhogam candidates (females of child bearing age that typed rh negative). The patients on that list are evaluated to see if they were rhogam candidates and if the workup has been ordered (if necessary) or dispensed. Our L&D and ED have very little problem with the process. There is the ocassional missed dose or inappropriate dose in ED but that is relatively rare and has been the same as before moving it to pharmacy.
My experience is that it is better in pharmacy than blood bank. Your mileage may vary.
-
On 10/25/2019 at 12:32 PM, Mabel Adams said:
At the recent AABB meeting San Antonio reported on their program with whole blood out on ambulances and helicopters. When the blood bags come in with the patient to their ED, they are sent to BB so they can be crossmatched after the fact. Here, we keep segments from the units that we provide to our medical transport partner so we can crossmatch those brought to our facility if serological XM is needed or if a reaction occurs. In TX they have two level 1 trauma centers and I think more than one supplier for the units so they have to try to solve it at the receiving site.
I work in a San Antonio hospital and what was reported at AABB was the expectation. It is NOT the reality.
First, all units supplied to the ambulances and helicopters come from our regional donor center. No hospital transfusion service provides the units nor are we responsible for the disposition of the same. All of this is monitored and tracked by the regional donor center and the EMS.
What is supposed to happen is, when a patient has been transfused en route, the completed unit and EMS's transfusion form is supposed to be sent to the blood bank upon arrival. This almost never happens. The only way we know in the blood bank is when we get mixed field reactions on our blood type or by reading physician notes on the chart. I read the physician note of every emergency release. That is how I normally find them. The physician will dictate that the patient received blood in the ambulance. It takes days for me to track down the paperwork and the empty bag is long gone by then. We NEVER crossmatch them because we never receive them.
All that said to say, your helicopter company needs to contract with whomever your blood supplier is to stock the units. That should not come from the transfusion services department of the local hospital. You might serve as a pass-through site, meaning the donor center uses you as a pick-up point but the units are never part of your inventory.
-
On 8/18/2021 at 8:13 AM, RKB1988 said:
Thank you all for your responses. There is a big risk in the Surgery department when it comes to blood requests. There is a significant amount of movement of patients and patient labels being left in rooms, paper charts etc. and request can me made on the wrong patient. It sounds like those that have responded do not have any provisions for surgery except "downtime" process. Any other ideas??
O.R. has forever been, and forever will be the weakest link in transfusion services. As was previously stated, there are a lot of moving parts and many of those vital parts have no computer access since they are contracted workers. For instance, the perfusionists and anesthesiologist have no computer access since they are only here for the case. All orders are verbal orders. They are the ones starting and ending the transfusions. There is no computer in the suite, everything is done at the desk by the circulating nurse. We have fought for decades to change that process but have had no success. Everything is manual in O.R.
- RKB1988 and John C. Staley
-
 2
2
-
Awesome site Malcolm. I was just reading the 1668 letter Acton, G. (1668) Physical reflections upon a letter written by J. Denis, professor of philosophy and mathematicks, to Monsieur de Montmor, counsellor to the French King, and Master of Requests concerning a new way of curing sundry diseases by transfusion of blood. London. Early English Books Online Text Creation Partnership. https://quod.lib.umich.edu/e/eebo/A26307.0001.001?rgn=main;view=fulltext.
Wow! How far transfusion medicine has come. I am glad I was born in the 20th century instead of the 17th century.
-
I agree with Malcolm. I haven't worked in a blood bank that routinely performs IS, or 37C readings in a long time. None of the instrument manufacturers antibody screens include them either. Your supervisor is helping you. You should thank her for saving you unnecessary work.
-
We do about 6 NICU transfusions/year. We routinely keep a fresh, CMV=, IRRADIATED unit on hand which is replaced every week. That unit is for emergency transfusion only in the NICU. If the situation is not critical, we order a fresh unit for the baby with satellite bags attached. It takes our supplier about 90 minutes to get that unit to us. The majority of our babies can wait. If it is an emergency, then we give the best product we have. The neonatologist says "we can treat hyperkalemia but we can't treat death."
-
We do not unless the patient has Anti-K. Darzalex is just a transient interfering substance. If there is no DTT neutralization required and no antibody detected, it is not necessary. Plus, I don't see how I can charge for the antigen typing in that scenario. I think that risks fraudulent billing.
-
I agree with David. Pick-up slips information and blood orders are all electronic and maintained in the LIS or the donor centers order website. CAP requires 10 years for blood and inventory inspection records, which includes shipping documents, since they usually contain a message about shipping, storage, and inspection.
-
BB.rick, I would have no problem retiring the card system in your case provided that you are regularly (daily) downloading a copy of the records to a secure, accessible file for downtime. Also, make sure all staff know how to access that data for prolonged downtime. There is no reason to have a card system with Meditech.
BTW, my previous position was in a place where the manager refused to stop using the card system. It was mainly because they were not familiar with how to access the information during Meditech downtime. Even when shown how, they still refused. It is still in use today at that site. Some battles you can't win.
- AuntiS, John C. Staley and Ensis01
-
 3
3
-
In the USA, there is an extreme shortage of techs that are blood bank competent. The ones who are are working regular overtime to fill in the shifts to provide 24/7 coverage. They are normally working at least two jobs. The field is facing a crisis as these techs who are qualified are now retiring. We just lost two of our best this year and are struggling to keep the younger, promising techs in the field. I'm afraid you won't really have a choice soon. You will have to take what you can get tech-wise and train them as best you can.
- Sonya Martinez and BldBnker
-
 2
2
-
On 4/27/2021 at 7:04 AM, kaleigh said:
Hi everyone,
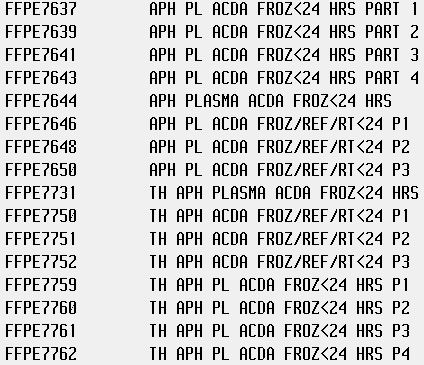
I am building some new Apheresis Plasma product codes into our LIS, and am trying to figure out which ISBT code I can use for the 5-day expiration "Thawed Plasma" labels. Can I just use E2121 (Thawed Apheresis PLASMA/ACD-A/XX/refg) for all of them?
For reference, here are the products:
E4689 – Apheresis FRESH FROZEN PLASMA|ACD-A/XX/<=-18C|1st Container
E4693 – Apheresis FRESH FROZEN PLASMA|ACD-A/XX/<=-18C|2nd Container
E4697 – Apheresis FRESH FROZEN PLASMA|ACD-A/XX/<=-18C|3rd Container
E4701 – Apheresis FRESH FROZEN PLASMA|ACD-A/XX/<=-18C|4th Container
E7637 – Apheresis PLASMA|ACD-A/XX/<=-18C|Frozen <=24h|1st Container
E7639 – Apheresis PLASMA|ACD-A/XX/<=-18C|Frozen <=24h|2nd Container
E7641 – Apheresis PLASMA|ACD-A/XX/<=-18C|Frozen <=24h|3rd Container
E7643 – Apheresis PLASMA|ACD-A/XX/<=-18C|Frozen <=24h|4th Container
E0869 – Apheresis FRESH FROZEN PLASMA|ACD-A/XX/<=-18C
E1624 – Apheresis PLASMA|ACD-A/XX/<=-18C|Frozen <=24h
Any help with this is so appreciated! Thanks in advance

You cannot use E2121 for all of them. Each of those frozen products have a corresponding thawed 24hour and a thawed 5 day plasma code of their own. You have to use those. We go straight from frozen to 5 day thawed so we only use those two. Here is a screen shot of our database.

- David Saikin and kaleigh
-
 2
2
-
CAP competency requirement is labor intensive, regardless of how you document. I have been trying to put more on their competency assessment tool they offer. Setting that up alone is labor intensive. It is the number 1 cited issue of most labs in America. It is not that we are not competent, of course. We have a hard time showing it the way CAP wants it. It really requires a full time QA position just to keep up with the competencies of all the staff in every department.
-
-
18 hours ago, Sonya Martinez said:
We recently switched to MaxQ coolers which are pre-validated by the company at storage temp. Then we validated at transport temp and storage temp and use that for when the cooler needs to be returned for fresh ice. Our policy specifically states this and that we consider them transport coolers. We really like the MaxQ coolers and have seen a huge decrease in waste due to temp issues on return of the coolers. The lids close by themselves. They even have coolers that have temp monitoring integrated but they're super expensive.
We just switched to the MaxQ MTP coolers and love them! My validations showed it held temps for 24 hours, even when opening the lid every 15 minutes for the first 2 hours and hourly after that. Plus, we filled the cooler with warm FFP (4 units @37C) and cold RBC (4 units @4C). The cooler cooled down the FFP units to 6C within 3 hours. The RBC'S never went above 5C.
- Sonya Martinez and AMcCord
-
 2
2
-
23 hours ago, Joanne P. Scannell said:
I'm also wondering how one manages to validate that all units of blood remain within temperature range when the ambient temperature and handling is not consistent.
We can't even validate our coolers for the same reason ... and one never knows if the cooler is left open or the units are removed then replaced.
Are you using 1-10oC or 1-6oC?
FDA instructed us to use 1-6oC for the coolers because they are really 'in storage'.
If not in a cooler, we can go up to 10oC because they are 'in transit'. I haven't implemented that part yet, but I will be soon.
We use 1-6 for coolers, however, the BT-10 only shows breach above 10 so, there's that. We place a NIST certified thermometer in the cooler to show that it is 6 or below upon return. We do not scan these units with the temp gun.
It's 1-10 for anything returned not in a cooler.
-
-
2 minutes ago, galvania said:
well yes and no. It would depend what the test was. The typical test not offering controls would be an antibody screen. As most of these are negative or positive with only some of the cells, this acts as its own control. Where you would need to perform a 'negative' control is if all screening cells are positive. This would be done by carrying out an auto-control with the panel. If that is positive too, however, you still won't know, in the absence of any other information, whether the patient has a true auto-antibody or whether the patient is reacting because of the potentiator in the AHG / diluent.
For antigen testing, a must however, especially if tested in an IAT or with enzymes
I believe he is asking about blood grouping. That is what the title of the message suggest, "Use of Negative Control in Blood Grouping."
I'm so confused.
-
I don't follow. Like you said, all the available automated platforms incorporate a monoclonal control in blood typing. Are you suggesting removing the monoclonal control from the blood grouping test on automated platforms?




Patient with WAA unable to determine ABO & Rh type
in Transfusion Services
Posted · Edited by jayinsat
punctuation error.
Hi Malcolm,
What terminology is recommended in these situations? We have always used "least incompatible" in the states. I think, probably, the majority of our databases have that option listed besides "compatible" and "incompatible." What terminology should replace "least incompatible?"