adiescast
Members
-
Joined
-
Last visited
-
Country
United States
-
-
-
-
Gold Medal.
Congratulations Malcolm! You have every right to be excited about this honor. Thanks for letting us know about it!
-
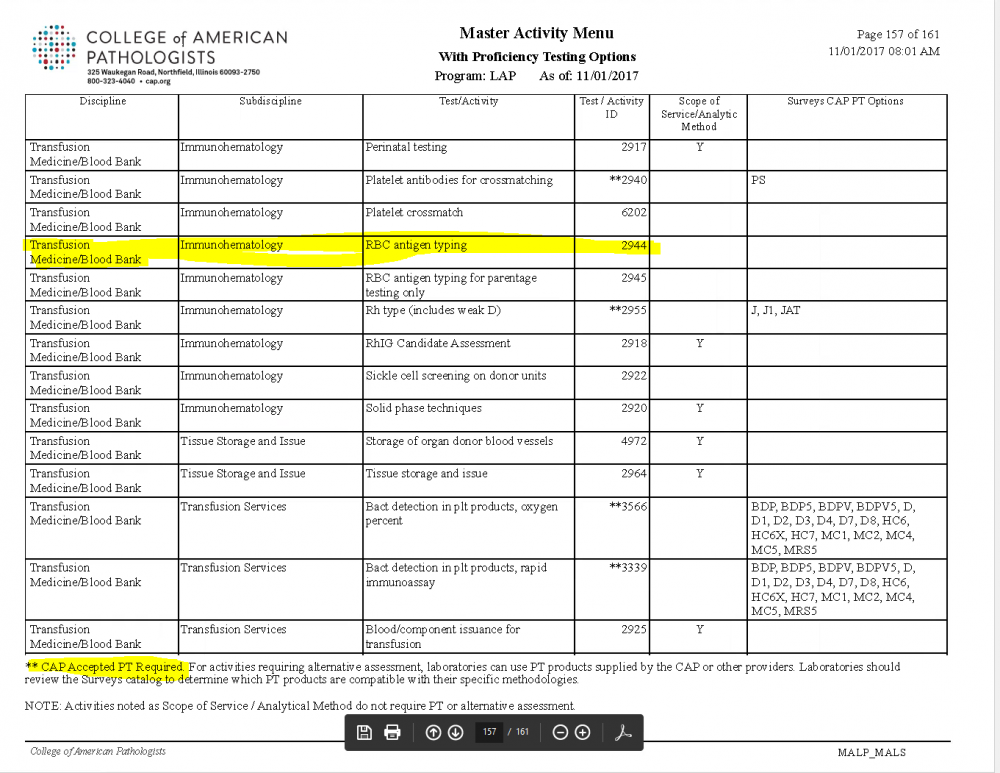
Antigen Typing Alternate Proficiency
The RBCAT survey is not required. The CAP Master Activity List does not include antigen typing as a test requiring CAP approved survey materials, so alternate PT methods are acceptable. Here is the checklist requirement: **REVISED** 08/21/2017 COM.01500 Alternative Performance Assessment Phase II For tests for which CAP does not require proficiency testing (PT), the laboratory at least semi-annually exercises an alternative performance assessment system for determining the reliability of analytic testing. NOTE 1: Appropriate alternative performance assessment procedures include participation in an external PT program not required by CAP; participation in an ungraded/educational PT program; split sample analysis with referral or other laboratories, split sample analysis with an established in-house method, use of assayed materials, clinical validation by chart review, or other suitable and documented means. It is the responsibility of the laboratory director to define such alternative assessment procedures and the criteria for successful performance in accordance with good clinical and scientific laboratory practice. NOTE 2: For in situ hybridization testing and other complex molecular and sequencing-based tests (including but not limited to microarray-based tests, multiplex PCR-based tests, and next generation sequencing-based tests), alternative assessment may be performed by method or specimen type rather than for each analyte or tested abnormality. For tests such as allergen testing, alternative assessment may be performed in batches of analogous tests. NOTE 3: Semiannual alternative performance assessment must be performed on tests for which external PT is not available. NOTE 4: This checklist requirement applies to both waived and nonwaived tests. The list of analytes for which CAP requires enrollment and participation in a CAP-accepted PT program is available on the CAP website [http://www.cap.org/] through e-LAB Solutions Suite under CAP Accreditation Resources, Master Activity Menu Reports. Also, the inspection packet includes a report with this information for each laboratory section/department. Evidence of Compliance: ✓ List of tests defined by the laboratory as requiring alternative assessments AND ✓ Records of those assessments . The Master Activity page is attached. Only items with the ** require CAP approved PT materials.
-
-
-
-
-
-
DARATUMUMAB
Our problem has been that these patients have been transfused before we get them, so we can't get a phenotype.
-
bleach in the blood bank
We run our saline bottles through the dishwasher process that is used for Chemistry's glassware. We have not had any issues with contamination.
- Safe-T-Vue's
-
-
Extra "just in case" specimens - How do you handle them
We also have a specific test in the LIS that we order for extra tubes (called "save") that lists the color tubes that were received. These are placed in a rack in the refrigerator. We do have a separate "saveb" for tubes that come properly labelled for blood bank. Those extras are saved in a rack in blood bank. The blood bank extras are saved for 3 days. I think the lab saves are kept for 1 day. The LIS test makes it easier to locate the tube when the order shows up.
-
Verbal Orders for Blood/Blood Products
We start our MTP with a specific pager call, which triggers off a verbal order from the doctor (who is often hands deep at that point). There is a set pattern for how much of each product goes in to each "cycle" of the protocol. It is in the procedure and posted in blood bank. All they have to do is set off the pager to start it. They are expected to record the MTP order in the patient record, We get emergency release forms for all blood that is released without crossmatch, but we get a sample as early as possible in the process to switch over to crossmatched.
-
O Negative PRBC's with MedFlight
We require the same competency of the helicopter crew that we require of our other transfusing staff. They maintain the documentation.
-
O Negative PRBC's with MedFlight
We send 2 units of O Negatives with every flight. We use the Credo Coolers, which we validated to 36 hours at 1 - 6 C. We sign out the units for 12 hour intervals, give or take depending on their schedule. They switch out the frozen inserts when they come for the next set of units. They take 4 hour temperatures of the coolers using an inside/outside thermometer that does not require opening the cooler to read. The temperature records are stored in blood bank. We do put Safe-T-Vue indicators on the units, but that is more to show if the units were removed from the cooler and might have gone out of temperature (they are 1 - 10 C indicators). We do return the units to inventory if they are not used. We charge them to the helicopter company if they are wasted or taken to another hospital.
-
-
Blood Bank bands on patients drawn while in Surgery
They apply it before leaving the surgery room. Our reasoning is that as long as there is only one patient in the room and neither the patient nor the bracelet leaves the room until they are connected that the possibilities for error are small.
-
Intra and perioperative blood transfusion
When ours was outsourced (it has come back in house now), the medical director reviewed the SOPs before implementation and annually and all QC performed. We had a company representative report their QA data at the quarterly transfusion committee meetings.
-
Sahara Plasma Thawer
We use the Helmer and have had no problems with it. The overwrap bags hook to the top of the agitator to prevent water entering the bag (unless the bag has a hole in it) and keep the plasma unit upright. I have no experience with the Sahara.
-
Transfusing A2 patients
We often give type O to patients with anti-A1 mostly because it is quick and easy (and doesn't confuse the poor computer).
-
cold agglutinin for CABG patients
We do not perform cold agglutinins or a cold panel for patients going to CV surgery. If we find a strong cold reactive antibody in routine testing, we make the surgeon aware so they can make appropriate decisions.
-
Post-partum RhIG multiple doses
If the pharmacy is handling the RhIg, I have known them to combine the doses for IM injection to avoid multiple sticks. They can also give some in each hip to prevent too much fluid on one side. I think the IM injection helps prevent the side effects (except for the pain in the injection site).