Leaderboard
-
-
Points2,700Posts
-
-
Popular Content
Showing content with the highest reputation on 08/15/2017 in all areas
-
Giving O Pos PRBC's to a male JohnDoe during a Massive Transfusion.
I agree with all the above we have used O pos on all our massive transfusion patients and on any emergency release blood given to a male or female >50. We also use A FFP in any emergency release or massive transfusion patient. The only problems that we have run into as with any massive transfusion patient is how late we get the T/S and then the patient shows as an O pos and then after being in the hospital a while starts to show their true type. As far as antibodies, in an emergency situation there are risks, but death is what we are trying to prevent. We have not had very many patients that come back with antibodies and Anti-D isn't even usually the one made. More often than not it is a K or Duffy, possibly a Kidd.2 points
-
HDFN in Twins Clinical disparity
HDFN in Twins Clinical disparity
Malcolm Needs reacted to John Quigley for a post in a topic
1 pointWe wrote this case report up and it got published in Transfusion Medicine. I can email the paper if anyone is interested.1 point -
Electronic Temperature Monitoring
My thinking would be if the electronic monitoring system would go down for some reason, do I know that my alarms will still work at the correct temperature?1 point
-
Meditech - Hard stop during entry of unit to force documentation of visual inspection
Meditech - Hard stop during entry of unit to force documentation of visual inspection
mollyredone reacted to macarton for a post in a topic
1 pointto save into a Word document, click PrtScn, send to clipboard, then to put in document, click on spot you want it and right click and paste. On the popup boxes, if you want whole screen with the pop up, hit PrtScn, then cancel, then to put in document, click on spot you want it and right click and paste. You'll have to do some trimming then.1 point -
Antibody Titers Gel vs. Tube
1 pointI looked up Method 5-3 "Using Antibody Titration Studies to Assist in Early Detection of Hemolytic Disease of the Fetus and Newborn" in the AABB Technical Manual, 18 edition -- One of the NOTES states: "Do not use enhancement techniques [albumin, polyethylene glycol, low-ionic-strength saline (LISS)] or enzyme-treated red cells because falsely elevated titers may be obtained. Gel testing is not recommended." The next note states: "LISS should not be used as a diluent in titration studies; nonspecific uptake of globulins may occur in serum-LISS dilutions."1 point
-
Meditech - Hard stop during entry of unit to force documentation of visual inspection
BBK Product Dictionary -- page 3 -- UNIT SCREEN (about 1/3 of the way down the page on the left) -- look up the documentation (Shift+F8). "Order Screens are customer-defined queries created in the MIS Dictionaries." When we built our inspection statement, we made it so it would only accept "Y" as an answer -- thus the required inspection.1 point
-
Meditech - Hard stop during entry of unit to force documentation of visual inspection
Meditech - Hard stop during entry of unit to force documentation of visual inspection
AuntiS reacted to mollyredone for a post in a topic
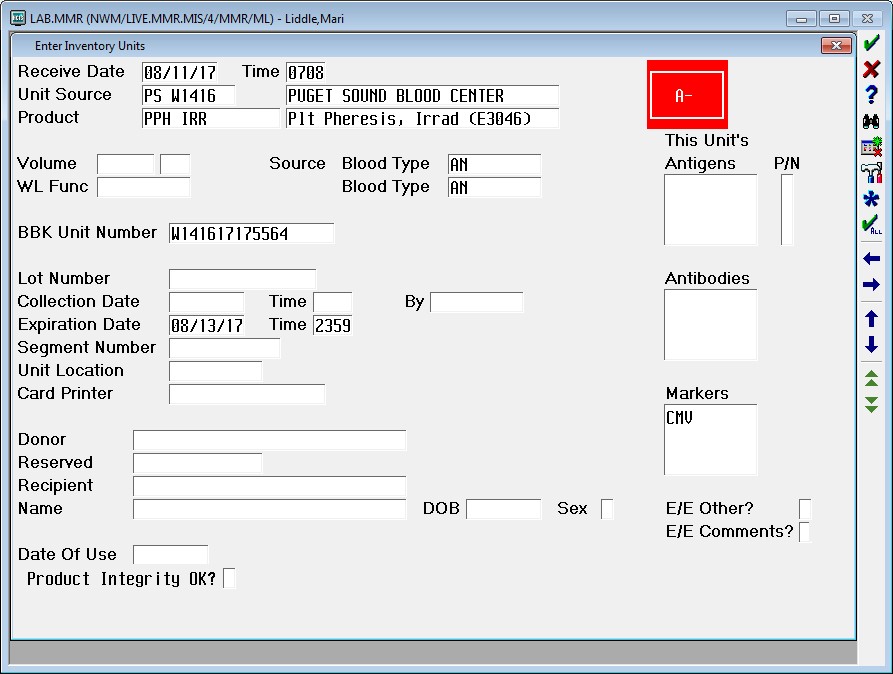
1 pointAuntiS, here's a screen shot of our enter units. The only difference is at the bottom under date of use. We have "product integrity OK?" and it is a required field. My IT guy says he thinks it is an NPR from Meditech. Maybe they have to do it, unless you have an IT guy that knows a lot about Meditech.1 point -
Antibody Titers Gel vs. Tube
Antibody Titers Gel vs. Tube
SarahE reacted to Mabel Adams for a post in a topic
1 pointAnother caveat about doing titrations on the Vision is that it always runs all 10 (or 12?) dilutions. That will burn through a lot of reagent cells unnecessarily on a titer of 4! I agree with those above that it is critical that the OB/GYNs know that you are using a method that gives different results than their textbooks are based on. Every gel titer result should go out with a comment explaining how its results correlate to the literature for further evaluation of the pregnant person. At least nowadays they are likely to follow with Doppler ultrasounds rather than riskier, invasive amniocentesis. I think a review of the CAP survey results is very enlightening.1 point -
Antibody Titers Gel vs. Tube
1 pointI am looking at the Participant Summary for latest CAP proficiency, anti-D titer. For tube testing using the "uniform procedure tube method" that CAP suggests, they reported the following results: 333 participants, mode 64, consensus range 16 to 256. For Gel testing "uniform procedure gel method", 138 participants, mode 256, consensus range 64 to 1024. Per CAP, Consensus is determined by the Mode +/- two of the most frequent titers. It looks like in the previous 2 surveys for the gel anti-D titers, the mode for gel was 1 to 2 dilutions higher than tube, but so was the consensus range. It seems like there are a fair number of labs reporting gel and if you report that as your method you should be compared to other gel titer users. I would also think as more instruments are implemented that can do gel titers, the number reporting gel will go up. And of course what ever you do, perform method correlation and communicate with the obstetricians any changes they may see in titer results. We are contemplating this also. Also I am little confused by ""Do you want it to be faster and more hands-off or more exact?" It seems to me that automated titers in gel should be much more reproducible. We are just starting to look in to performing titers on Vision.1 point
-
Antibody Titers Gel vs. Tube
Antibody Titers Gel vs. Tube
David Saikin reacted to jalomahe for a post in a topic
1 pointWe do our titers in tube. Years back when a lot of places had switched or were getting ready to switch to gel there was a conversation about the difference in titers due to sensitivity of gel. The basic conclusion at least in our geographical patient care area: we didn't want physicians to be getting different titers from different labs solely due to differing methodologies as it could lead to unnecessary concern/procedures for the patient. So we all stick with tube method. In those instances where we detect the antibody by a more sensitive method i.e. gel or Capture but the titer is negative then we report the antibody titer as less than 1 (<1). Jan1 point -
Antibody Titers Gel vs. Tube
1 pointWe were doing titers in the gel and kept failing our CAP surveys for titers. They were always one titer too high (CAP gives you a 3 titer range the results can be within to pass). After investigation (I was new at this job at the time) I found where the Technical Manual says that they shouldn't be performed in gel. (Like SMILLER says above). It goes on to state that there is a danger with interpretation by the physician and the higher results of unnecessary invasive procedures performed due to that combination. They, strangely, do not tell what studies they base that comment on. We switched to the tube method and haven't failed a survey since. We are in the process of deciding on new Blood Bank automation. When we saw the Vision, that seemed to be one of the "hot" selling points - the ability to perform titers. We questioned them about how they felt about it being contrary to AABB recommendations to do titers in the gel. The response was something like, "Do you want it to be faster and more hands-off or more exact? We would rather see it be more hands-off." (Not a direct quote, but you get the picture.) Personally, I would prefer the "exact" results to one that may or may not be too high. I would also prefer to pass my CAP competency surveys! Just saying.1 point
-
Antibody Titers Gel vs. Tube
1 pointWe are a small facility which uses manual gel technology. We rarely see titers ordered. However, when reporting an antibody on a pregnant patient (detected and identified in gel) we faced this dilemma: the antibody was so weak it was not detectable in tube. We then started using gel for titers with the understanding that it is the change in titer over time that is significant. For proficiency, we use the split sample method every 6 months. CAP and State (NJ) inspectors have been OK with this.1 point
-
Antibody Titers Gel vs. Tube
1 pointOn p. 563 of AABB Tech Manual 18th edition, it only mentions that titer methods other than "saline AHG 60 minute incubation" in tube may result in higher titers and "should be validated with clinical findings" (see Malcolm's post, above). So it does not seem to say one cannot use gel or other methods, just that you need to document validation. I have always been a bit uncomfortable with identifying an antibody with gel (for a prenatal), then doing the titers in tube. But then again, I guess it is the comparison of the series of tube titers that they are looking at. Scott1 point
-
Antibody Titers Gel vs. Tube
1 pointThe AABB Technical Manual states that antibody titers should not be performed using gel technology, so we revised our procedure to go back to tube method.1 point
-
Giving O Pos PRBC's to a male JohnDoe during a Massive Transfusion.
Giving O Pos PRBC's to a male JohnDoe during a Massive Transfusion.
SMILLER reacted to Mabel Adams for a post in a topic
1 pointI agree with MaryPDX. We start with O pos for males and females over 50. Remember, 85% of them will turn out to be Rh pos. We don't switch to Rh neg for Rh neg patients who have already received Rh pos until the crisis is over; they might get Rh neg for a top-off the next day, say. We don't use type-specific uncrossmatched as a rule--too likely for someone to assume they don't need to check ID because it is uncrossmatched, in my book. We need a blood type on second specimen to give anything other than O anyway and by then the units will be crossmatched. There is too little plasma in modern RBC units to worry about switching back to type-specific. No special crossmatch required. We might on occasion phone the BB of the receiving hospital to give them a heads up that we have caused the patient to type strangely or that we found antibodies if we think it is information they would want to know. If you do blood types in gel, the mixed field is so striking it gets people's attention more than in tube testing. We only recently got an instrument that runs gel so we do gel blood types on it. Maybe we will get used to the mixed field reactions eventually.1 point -
Decoding AABB Std 8.2 on Blood Utilization Monitoring
At my hospital, we split out our Blood Utilization monitoring like this (below) to cover each of these 10 AABB requirements: 1. Ordering Practices: C/T Ratios (Total and by Service), Uncrossmatched Blood Requests, Compliance with MD signature completion on "Release of Uncrossmatched Blood" forms 2. Patient Identification: Any Patient ID issues identified are reported 3. Sample Collection and Labeling: BBK Specimen acceptability report (outliers are detailed) 4. Infectious and Noninfectious Adverse Events, Incidence of Mistransfusion: Transfusion Reactions, Recalls, Notifications 5. Near-Miss Events: Anything identified would be detailed along with root-cause, corrective action 6. Usage and Discard: Units transfused, Units discarded 7. Appropriateness of Use (for all Components): QA Audits performed by Medical Director, report pulls 100% products transfused (one type of product per month) and the most recent related lab data to when the product was issued. Outliers are investigated by the Medical Director. MDs receive follow-up letters on questionable transfusions. Jan/Apr/Jul/Oct = RBC compared to most recent Hgb/Hct Feb/May/Aug/Nov = PLT compared to most recent PLT count Mar/Jun/Sep/Dec = FFP and CRYO compared to most recent PT/PTT and Fibrinogen We also review any Autologous transfusions, Peri-Op Collection/Reinfusion (Cell Saver, Hemodilution, ECMO) and Therapeutic Apheresis and Phlebotomies performed during the quarter. 8. Blood Administration Policies and Practices: Our QA department performs random audits on 75 transfusions per quarter (coordinated with the RBC, PLT, and FFP/CRYO schedule above) and audits for MD Order to Transfuse, Issue Checks Performed, Bedside Checks Performed, Vitals Recorded. Since we are using TAR, all transfusion data is now entered into the computer so this audit can be done using computer reports. We also audit 100% of all transfusions for "Duration Less than 4 Hours" by running a computer report. We also report on Bedside Blood Administration Audits which are performed by the blood bank techs (minimum of 16 per year) and Blood Warmer QC which is performed quarterly by Biomed. 9. Ability of Services to Meet Patient Needs: We do Turnaround Time studies (Type & Screen, DAT, Crossmatch, Newborn Workup), report on any Customer Complaints and Cancelled Treatments. We also have a Bloodless Medicine and Surgery program so we report on the number of patients enrolled in the program who were treated without transfusion. 10. Compliance with Peer Review Recommendations: Letters Written/Sent to Phsyicians from the Medical Director and/or Blood Utilization Committee Chair, and Responses Received I know, some of these do overlap somewhat, but "Ordering Practices" would be looking at how many and what kinds of products are doctors routinely ordering for various things (i.e. are they over-ordering or under-ordering for certain types of surgeries/procedures, do they order uncrossmatched too often because lack of ordering in advance) and "Appropriateness of Use" would be looking at whether they make the decision to actually transfuse those ordered products to their patients based on appropriate criteria (lab results, patient symptoms, etc.).1 point