jalomahe
Members - Bounced Email
-
Joined
-
Last visited
-
Country
United States
-
-
-
OCTAPLAS
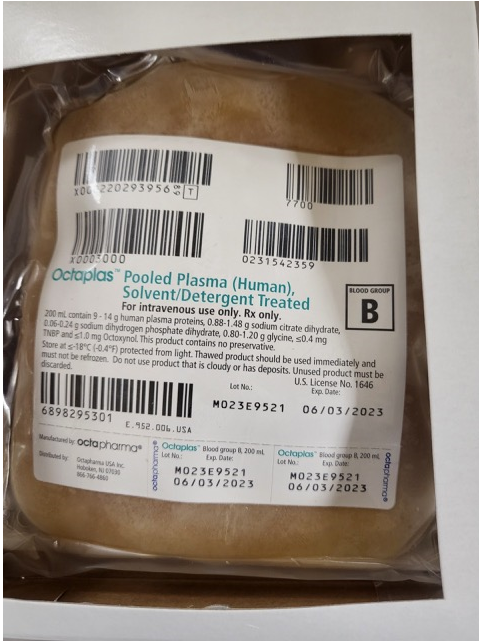
Anyone have any experience with OCTAPLAS - Pooled Plasma S/D (Human) ? Our hospital pharmacy is inquiring as to whether it should be handled by them (pharmacy) or by the transfusion service/blood bank. If you handle it in the blood bank do you have it set up in your computer systems same as an FFP and do you know the Product code for it? The example unit (see attachment) that I saw had a face label "similar" to an FFP but without the corresponding product codes so would we have to reprint a face label and relabel the stored frozen product? What about the thawed product? TIA
-
-
Can Blood Bank Share a Refrigerated Centrifuge with Micro?
I would do a couple of things 1) Check with you infection control department and get their input 2) Check with whatever agencies do your inspections: AABB, CAP, FDA etc to see if this is something that they would cite you for on next inspection. If all of those folks are okay with it then I think you can move forward with it.
-
-
-
-
-
-
Labeling testing tubes/wells
Patient first and last initials and reagent in the tube so for blood type it would be: JH-A, JH-B, JH-D, JH-DC for the front type and JH-AC, JH-BC for the reverse type.
-
COM.30840 Pipette Carryover
For everyone on here with automated blood bank equipment especially Immucor Echo: what do you have in place for this CAP requirement? Do you make a high titer specimen to run? How high of a titer and what do use to make it? Immucor studies for Echo show that carryover does not occur with an antibody titer up to 5120 so where should my titer point be for the requirement? I'm trying to think of a way to do this without making myself and everyone else in the department crazy. COM.30840 Pipette Carryover Phase The laboratory evaluates its automatic pipetting systems for carryover. NOTE: The laboratory must have written procedures for evaluating whether carryover effects are present. This requirement applies to both stand-alone pipette systems and to sample pipettes integrated with analytic instruments. One suggested method to study carryover is to run known high patient samples, followed by known low samples to see if the results of the low-level material are affected. If carryover is detected, the laboratory must determine the analyte concentration above which subsequent samples may be affected, and define this value in the procedure. Results of each analytical run must be reviewed to ensure that no results exceed this level. If results that exceed the defined level are detected, then the appropriate course of action must be defined (repeat analysis of subsequent samples, for example). Carryover studies must be performed, as applicable, as part of the initial evaluation of an instrument. (The laboratory may use the data from carryover studies performed by instrument manufacturers, as appropriate.) Carryover studies should be repeated after major maintenance or repair of the pipetting assembly of the instrument. Evaluation for carryover is not required for automatic pipettes that use disposable tips. This requirement is not applicable to coagulation. Evidence of Compliance: Record of carryover studies at defined frequency
-
-
-
-
-
-
-
-
-
Inappropriate Override by a Manager
If you feel that you are not being heard or appropriate action is not being taken you have the option of reporting to your accrediting agency AABB, CAP, TJC. This can also be done anonymously. The agency will contact the lab for information/investigation. If patient safety is being put at risk then you need to do everything you can to get these situations addressed which it sounds like you are doing. The other thing I would highly recommend is that you keep written/hard copy documentation of everything. You may need it sometime in the future to have proof of your diligence in trying to force compliance. Always remember the old adage "If it's not in writing, it never happened" Good luck
-
COBE Cell Washer QC
If the manufacture has no recommendations I'm not sure that this is a necessary QC/PM process. I've had inspectors suggest something because it's what they do at their lab but that doesn't make it something that is required to be done. How long has this protein check been being performed and has it ever been positive? Wouldn't the presence of excess protein cause the Check Cells to not work when added to negatives? If your Check Cells are working during daily reagent QC and whenever you do DATs and/or IATs I don't see the point of the quarterly test for protein. Now if you were having a problem with Check Cells not working I would think checking for excess protein as a trouble shooting option would be worthwhile.
-
-
- TAT Quality Monitors
-
-
-
Cardiac surgery and what it means for our blood bank?
When we brought cardiac surgery on-line at our facility many years ago I made sure that I was included in planning meetings with the Cardiothoracic teams that way I didn't get blindsided with their expectations. Our cell savers are run/managed by the perfusion team with oversight by the hospital Blood Utilization Committee and Transfusion Safety Officer. Our MSBO has only a TYSC for OH surgeries. They do not always need blood products depending on the extent of the surgery. It's not unusual for the surgeon to want 2 RBCs available in surgery but patient may or may not get them. We also get requests for 2-4 FFP, 1-2 pooled Cryo (5u/pool) and 1 dose of platelets (we keep single donor Pheresed on hand) for the patient when they are bringing them off of By-Pass. They are usually good about giving us a heads up of when to thaw so we don't end up thawing too early or have them wasted if they decide later they won't need them after all.
-
Anti f?
Thanks Malcolm. I don't know why but f has always confused me way more than it should and now that manufacturer's have taken it off the panel antigrams it becomes an enigma that I have to try to explain to techs with less experience than I .....
-
Anti f?
Malcolm Either DcE/DcE or DcE/dcE
-
Anti f?
I have an B Pos prenatal patient who has a positive antibody screen by Capture Solid Phase. Testing shows reactions with rr cells only in both by Capture Solid Phase and IAT with PeG. I'm thinking it is likely to be anti f? Am I on the right track or is there something else I should consider?
-
Rh negative Patients that receive Rh positive blood
We have a policy to offer RhIg if we have given Rh Positive Random Donor Platelets to an Rh Neg female of childbearing potential. The amount of RBCs in a platelets is normally less than can be covered by a single dose of RhIg unless the platelets were particularly bloody in which case we don't accept them from our supplier. Luckily these days our supplier is doing a great job of supplying only Single Donor Platelets so bloody platelets are not an issue. Giving enough RhIg to cover a RBC transfusion would not be a reliable or economically feasible option since the RhIg we have on hand only covers 15mls pRBCs. So a one (1) standard unit of packed RBCs would take in excess of 18 doses. As Malcolm said giving the RhIg would have the added downside of clearing the Rh Pos RBCs you just gave so unless you have additional Rh Neg units available you are just putting yourself behind again.
-
Nursing Order
FIRST SCENARIO: We have Epic as HIS and it has a module called BPAM which allows patient/unit bedside scanning. When we receive order to Prepare product we complete the order and it available for nursing to see that it is ready in the EMR. When nursing is ready to Transfuse they must "release" the Transfuse order in Epic. A copy of the released Transfuse order that includes patient id'ing information and the component and any special requirements. If we don't get the released Transfuse order we don't issue the unit MAINLY because, if they didn't do the release correctly they will not be able to scan the unit into the EMR at the bedside hence delaying the transfusion and possibly wasting the unit. SECOND SCENARIO: We frequently get Prepare orders but the unit is never transfused. That is not an issue for us since it doesn't affect our inventory in any way. We do mainly computer assisted crossmatches so we don't actually "crossmatch" the unit to the patient until the do the release Transfusion order (see above). The only crossmatches we do at time of Prepare order are those patients who don't qualify for computer assisted crossmatch i.e. have antibodies. If there was an issue as you described above I would write up a Safety/QA report and as long as you were following SOP then the onus for transfusion errors is on nursing not blood bank especially if you cannot see the orders.
-
Epic and blood bank
We have Epic 2018a with Sunquest v8.0. They are working well together but always a work in progress. Nursing is using Epic BPAM so they are doing barcode scanning of the units at bedside so we are now able to issue blood product without any paperwork other than the patient/unit identification tag on the unit. We are in the process of piloting the same process of blood transfused in the OR given by Anesthesiologists. Next up is creating process for rapid scanning during Massive Transfusion.
-
Massive Transfusion and Incompatible Plasma
consult with pathologist and keep in mind If you absolutely have to give incompatible plasma the ideal is to give it while patient is actively bleeding. If possible give RBCs that will be compatible with patient and the plasma so in your case O and as the bleeding is beginning to come under control start giving ABO compatible plasma to "top them off". The idea is that as long as patient is actively bleeding give them the incompatible product which is then being bled out onto the floor or wherever. Once bleeding is under control give the good stuff to help dilute the incompatible out and leave them with the most compatible antigen/antibody combinations possible.