Everything posted by Sonya Martinez
-
Infant transfusion units
Although I agree with @Neil Blumberg regarding CMV seronegative blood we had 2 heart transplant candidates patients in our Cardio Thoracic ICU that were originally CMV negative by pcr and came up CMV positive while inpatients and both had severe infections. Our infectious disease physicians decided it was due to the multiple blood and platelet transfusions (both were put on ECMO and had multiple surgeries) and now they order CMV seronegative products on all heart transplant candidates regardless of CMV status and those with DiGeorge Syndrome (due to their immunodeficiency - per my med dir not CTICU). They refuse to listen to reason or science and they didn't even bother testing any of the staff, parents, or other people working in the unit. (This is me rolling my eyes). Our bone marrow and stem cell transplants don't require CMV negative products but the kids in CTICU do. As far as transfusing pRBCs, we wash (and waste) a considerable amount of RBCs for any <4 months old, < 4 kg neonate that is also going through open belly surgery, open chest procedure (not on CPB), kids with K issues that have current elevated K+ levels, HUS due to Strep pneumo (and before ID of the cause). Our heart room washes the blood during CPB so we don't have to. We also have a <6 day old RBC, <24 hour irradiated, policy to keep the K down if >4 months but <1 year or >4 kg but it's for surgeries and any transfusion >20cc/kg. Most normal, non-surgical transfusions for neonates are done with irradiated, CPDA-1 or AS-3 RBCs but the volume is normally 10cc/kg at a rate of 2-5 mL/kg/hr. In order to keep the patient on the same unit as long as possible we normally start with 6-10 day old unit as well. I really don't want to switch to washing for all neonatal transfusions. Our wastage rates are already too high.
-
What brand of cell panels do you use for antibody identification (0,8-1%)?
We use BioRad for all our gel supplies but I know Grifols has gel supplies as well. We also use Quotient's 16 cell 3-5% panel which is normally outstanding for rule outs.
-
Liquid Plasma
According to the Circular of Information Liquid Plasma contains viable lymphocytes. It is plasma separated directly from whole blood. I don't see anywhere where it tells you the number of viable lymphocytes.
-
Validation of irradiator
In 2016 we paid around $250K for a Rad Source 3400 Blood X-ray Irradiator with installation and earthquake bracking plus we pay around $13K for annual PM and services calls (parts, tech and travel included). They're service is top notch and we're never down for more than 24 hours and it's a workhorse that can irradiate 12 units at once (2 per canister with capacity to irradiate 6 canisters at once). Oh and you can irradiate blood and platelets at the same time just not in the same canister. The newer models have a much smaller footprint than the one we have. https://www.radsource.com/ No I'm not a spokes person I just really love our irradiator!!
-
CAP staff competencies
Has anyone used WellSky's Quality and Competency Suite (used to be KnowledgeTrak) for competency and direct observation tracking and completion not just for a blood bank but for an entire hospital laboratory? I've used WellSky's Transfusion since 2013 and I had them do a couple of demos for us but the others in lab leadership are wanting to go with MediaLab plus CAP's Competency Assessment Program. However, it seems like there's a lot of build we would have to do on our own for CAP's tool whereas after I gave most of our stuff to WellSky they've already built in what we currently utilize plus they have staff that can help us with the build needed. I did like MediaLab's document control system (our hospital wide one is crap and has resulted in multiple citations from CAP and AABB) and their Inspection stuff but with AABB's APEX site would that mean duplicate work?
-
Exchange Transfusions for babies
Here's ours. Hope it helps. You can disregard the last part with the computer entry unless you use WellSky Transfusion. BBI0023 Exchange Transfusion 040221.docx
-
Temperature Indicators
Hi Justine, We switched from the Safe-T-Vues a few years ago for the same reasons. We use the Blood Temp 10s in our coolers (we validated for transport not storage) now. They are great and easy to validate with the coolers. The only thing is you have to make sure to place the indicator in the middle of the bag (on the back) and not the top or bottom because it can activate if there's no enough blood under the indicator. We've had problems with our washed RBC units and small volumes in transfer bags if they're not folded properly before going into the cooler. But I can't imagine having the same problem with whole blood. You can also get a temperature monitored cooler from MaxQ (they come pre-validated) https://www.packmaxq.com/ The Max Plus Alpha 2.0 has continuous temperature monitoring but I think it's only good for 24 hours. There may be other continuously monitored coolers out there we just use the MaxQ coolers so I have experience.
-
FDA verax testing day 4
Both our vendors are collecting either LVDS (large volume delayed sampling) apheresis platelets or Pathogen Reduced or both so we aren't implementing the Verax testing. We do, however, accept <3E11 platelets (low volume) LVDS if there's no other option. Implementing the Verax testing would be really complicated because 80-90% of our platelets are split at least once due to the size of our patients/children.
-
Emergency Release Physician Signature in EPIC
Applejw, what tests/products are in your Emergency Released order set? If possible can you share your SOP? Thanks
-
Any Level 1 with Epic / Wellsky combo?
We've been on WellSky Transfusion (formerly Mediware) since 2013 when we went live with Epic Beaker as well. We're a 300+ bed (at main hospital) children's hospital with a level 1 trauma, bone marrow transplants, extremely busy open heart surgery program plus liver, heart and kidney transplants. We went live with WS Transfusion web based 2020 R3 in April. The only thing we haven't instituted is Blood Product Administration Module with Matching in Epic but we're working on that now. If you need anything let me know, samartinez@rchsd.org
-
Infant transfusion units
For us, we don't consider the unit fresh if it's been irradiated more than 24 hours but only if the transfusion is >20cc/kg or push transfusion. Those get rotated back into normal stock and used for all patients including neonates as long as the volume is <20cc/kg.
-
Pediatric and neonatal transfusion considerations
Working in a children's hospital we have MTP set up by weight (which is either taken by the trauma bed or guessed by MD). We only give O NEG RBC, AB FFP and AB NEG platelets to females, males are allowed AB POS platelets without MD notification but anything else needs to be approved by pathologist on call and trauma MD or surgeon: <20 kg: 1 full RBC prefer fresh <7 days CPDA-1 or AS3 or whatever we have on hand if no other option, 1 <200ml FFP or 1 AB NEG liquid plasma and 60cc of AB NEG apheresis platelets (LVDS, PRT or other apheresis platelet including low volume - with less platelets in the original bag). 20-49kg: 2 RBC, 2 FFP (200-400mL) or 1 liquid plasma + 1 FFP or 2 liquid plasma, 1/2 unit platelets >50kg: 4 RBC, 4 FFP (start with 2 liquid plasma then switch to FFP, 1 full apheresis platelet We have been having a really hard time getting AB NEG liquid plasma so we're working on doing 5 day thawed plasma but it's a huge computer build. It's currently approved for MTP activation just like our liquid plasma, so we would start with 5 day thawed plasma and switch to FFP once it was thawed and ready that way we're not dealing with decreases in coag factors. According to the Tech Manual we're supposed to give ABO group specific or compatible platelets but it doesn't say anything about FFP compatibility. Since we deal a lot more in very small volume patients we choose to only use type AB if unknown type. I unfortunately don't have any other resources except the AABB 20th edition tech manual and Pediatric Transfusion Therapy (AABB publication from 2002). Hope this helps a little.
-
Billing for Washed RBCs
There is no HPCS code for washing RBCs. We bill for the procedure itself using CPT 86999 and for the RBC once transfused using P9016.
-
Isohemagglutinin titers
We have a very large heart transplant program which includes the possibility of giving an ABO incompatible (ABOi) heart to patients under 2 YO with low IgM and IgG Isohemagglutinin titers (generally <32). Luckily all but one of the patient's listed for ABOi transplant have been given either type specific or type compatible hearts. We have 4 different orders for isohemagglutinin titers; a simple backtype that we only perform during surgery to access the plasma and/or exchange transfusions by the perfusionists have dropped the antibody level down prior to the end of the transplant surgery, a 1:32 titer they can order if need a STAT result, a saline titer and an AHG titer. Both the saline titer and the AHG titer are done at listing, every month until transplant, immediately pre-transplant and daily for days 2-7 post-operatively. Recently we transplanted a patient listed for ABOi with a type specific, and therefore compatible, heart but our CTICU keeps ordering and collecting for the AHG titer for the last 4 days. I've been cancelling and stating in the cancellation comments the test is not necessary for ABO compatible heart transplants but nothing has changed. I personally don't believe there is and my medical director agrees with me. Is there some other reason to perform either the saline or AHG titer on this patient? (FYI: we do not currently have a blood or lab test utilization committee and I've contacted the CTICU NPs and MDs in hopes they'll cancel their standing order.)
-
Universal leukoreduction and ABO identical transfusions reduce HLA alloimmunization by transfusion to near zero
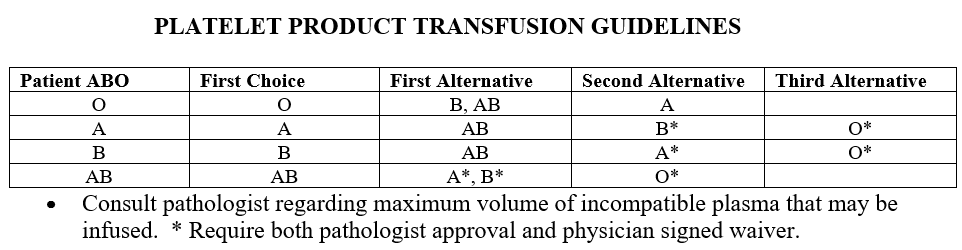
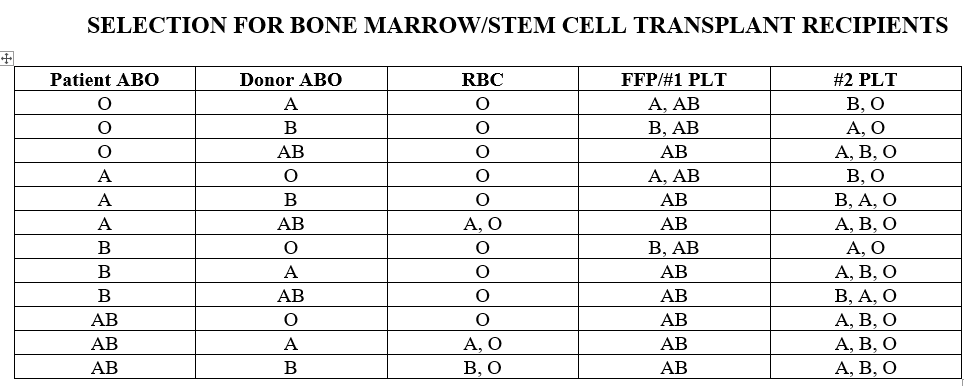
Working in a children's hospital we only give ABO compatible platelets or type AB but our first choice is identical. In most cases we use ABO identical and we have a very detailed Selection of Appropriate Component policy. For patients who received or will be receiving BM/HPC transplants we give compatible based on matching donor and recipient types, or type AB only (example patient type A receiving type O transplant gets either A or AB platelets). Also our listed ABO incompatible heart transplant candidates we will only give type O RBC and type AB plasma containing products until transplant and then ABO compatibility is determined by donor to recipient type. I attached our transfusion guidelines for non-transplant patients and BM/HPC transplants. It has been over 15 years since I've worked in an adult hospital so I'm not sure how hard it would be to implement our policies but I know we have a white board where we keep track of the platelets so we can easily look and make sure we have what we need at a glance.
-
Neonate Platelet Aliquots - references?
Hi lalamb That's a typing error, sorry. It should read we don't accept cold storage platelets. We didn't accept pathogen reduced platelets because our NICU was worried about the lower volume of platelets in each unit but we had them talk to those working at UCSD who's been 100% PRT for a while and they changed their minds. Then it was just getting the build done which is tedious and extremely time consuming in our computer system since we divide and change to an open code for syringing. Luckily we decided not to irradiate them or that would have been even more codes to build and validation!
-
Transfusion in surgery pediatric cardiac
For open heart surgery our perfusion team washes the red cells in the OR (faster than we can) and uses those with a small amount of FFP (for babies usually < 1 year especially those < 4kg). Our policy is to provide 1 fresh, <6 day old, irradiated (<24 hours) AS3, CPD, CPDA-1 or CP2D packed red cell for post CPB but we give then two <= 10 day old unit (irradiated, AS3, CPD, CPDA-1 or CP2D) and mark them "To Be Washed" for priming the CPB. For non-pump cardiac surgeries we wash if the patient is <4kg. I would love to get away from washing RBCs for surgeries (we also wash for major open belly procedures on <4kg infants) but over 20 years ago a patient died because of a K+ overload from a RBC that was irradiated 3 days prior to surgery even thought the unit was still <6 days old. Now I can't even get our Transfusion Committee to even discuss the topic. Guess I just wait until more surgeons retire.
-
Ortho MTS Gel Workstation Validation
Here's our plan from 2010 for the equipment validation. Hope this helps. Here's the variances we noted during the validation which you might need: The centrifuge will not spin if the drawer is not latched. Do not press the START button while the centrifuge drawer is open or the time display will automatically start counting down from 10 minutes. The centrifuge time is controlled by a pre-programmed timer that applies power to the motor for 10 minutes. Time and speed are pre-set at the factory and cannot be changed. If the START button is pressed with the centrifuge door open, the timer will automatically start counting down from zero, however, the motor will not start. Pressing the STOP button will clear the time display. Validation plan-MTS COMBO.doc
-
Weight-based pediatric blood orders
I agree with slsmith, we weigh each bag and write the volume on the unit or the transfusion record (we're still using paper). We don't have a set age where they can't order by volume so on some kids we end up doing a full unit plus a partial unit. For example if they order 425mL we give 1 full RBC that's 310mL + a partial that's 115mL. As far as setting infusion guidelines our standard is 1-2 units cryo per 10kg as fast as tolerated, 10-30mL/kg for FFP at a rate indicated by clinical situation (normally as fast as tolerate), 10 ml/kg RBC at a rate of 2-5mL/kg/hr or as ordered by physician, 5-10 mL/kg Platelets (we only use apheresis not WBD) as fast as tolerated and 10 mL/kg granulocytes over 4 hours.
-
Switching blood types in trauma situations
For the second ABO we use a specimen collected at a different time like a CBC but we won't use one collected at the same time as the type and screen. It's rare for us to request a specific draw for this testing but it does happen. We're a level 1 Trauma in a Children's Hospital but can take adults during disasters and pandemics (new one for me). We just changed our emergency issue and MTP policies adding in liquid plasma to start the MTP and we gave our computer the ability to give Rh POS to Rh NEG RBC and plasma products to males (policy stated if >1 year old with prior approval from pathologist on call and transfusing physician). We are only to use it in extreme situations where there's no other option. But with the working in AABB Standard 1.4.2 regarding product inventory shortages, 5.15.2.1 policy for use of RH pos RBC containing components to Rh Neg recipients I felt we didn't really have a choice. Luckily we have 1 primary blood supplier and a secondary contract for platelets and other products during emergent situations.
-
Thermometers for taking temp of returned blood products
Dave Saikin and JeanB. My policy actually lists that the MaxQ coolers are validated to hold a storage temp for X hours and a transport temp for X hours (dependent on each cooler) but we choose to call them transport coolers. All the coolers are back in 12 hours and their ice packs changed which is within the validated storage and transport temp so we should be safe. Also, FDA and AABB have both reviewed our policies prior to the use of MaxQ coolers multiple times and never said a word. I think it's the very lengthy validation we do at RT and >30C monitoring the temp inside the cooler, using BT10s, and each individual saline/glycerol bag every hour until it reaches 10C at max and min volumes. For the last 15 years I've been here we've always called them transport coolers.
-
Neonate Platelet Aliquots - references?
That's a great idea. Do you print the labels yourself or did you have them made by a printing company (like Shamrock)?
-
Neonate Platelet Aliquots - references?
lalamb: Sorry for the delayed response I'm going live with a computer upgrade this week and I've been neglecting everything else!! We will be accepting pathogen reduced platelets starting May 1. Mostly it's because our physicians on the transfusion committee are extremely conservative (and rather dated) and wanted more data. But we don't really have a choice since our secondary vendor for platelets is ARC and they are going 100% PRT later this year. Besides most of the other area hospitals that have NICUs and PICUs in SD county are run by Rady Children's Hospital and they use PRT now anyway. Lastly, our primary vendor collects more apheresis and just started their PRT collections recently. It will be interesting starting this weekend when we can accept them. I'm not looking forward to all the calls from nursing since the label doesn't actually state pathogen reduced. We'll see who reads their education and emails!!
-
neonatal transfusion
I wish we could get some of our physician's (heart transplant mostly) to understand that leukoreduced = CMV safe and that it should be equivalent to CMV NEG but they insist we keep giving CMV NEG RBCs. We still mostly get CPDA-1 RBCs (fresh, < 6 days old) but we added AS-3 to the list of what neonates can receive a couple of years ago when there was the shortage on CPDA-1 collection bags. So now we use CPDA-1 interchangeably with AS-3. It's weird because our HPC (including bone marrow out of ABO and/or Rh type) transplants don't get CMV NEG but our heart transplant candidates and recipients all do. Our CVICU (cardiovascular ICU) had 2-3 patients end up CMV POS and they refused to test staff saying it had to be from the blood and blood products. Then COVID hit and everyone started wearing masks 100% and no more CMV POS patients even though we didn't change from what we were giving.
-
Barrier method
We still use a clerical check on the paper Transfusion Record then all records come back to the blood bank for review. If they don't fill the clerical check on perfectly (2 medical staff signatures, full date and time completed) I put in a safety report. It's one of nursing's performance improvement plans to be >95% each month. I would rather they do the 'double check' in the computer but we don't have Epic BPAM (blood product admin module) set up to match the results filed when we issue the product to the barcodes they enter at the beside before transfusion.