NLiveris
Members
-
Joined
-
Last visited
-
Country
United States
-
-
Thawed Plasma ISBT Code
Hello Kaleigh, Per ICCBA, you will need to carry over the container attribute, therefore you cannot use E2121 for all of them. You can use E2121 as the 5-day Thawed Apheresis Plasma code for E0869 and E1624 (the last two on your list). Below are the thawed codes you need. E2121 = Thawed Apheresis PLASMA|ACD-A/XX/refg E5548 = Thawed Apheresis PLASMA|ACD-A/XX/refg|1st container E5549 = Thawed Apheresis PLASMA|ACD-A/XX/refg|2nd container E5550 = Thawed Apheresis PLASMA|ACD-A/XX/refg|3rd container I hope the above information helps. Thanks, Digi-Trax Corporation
-
-
ISBT-128 Diamond Symbol
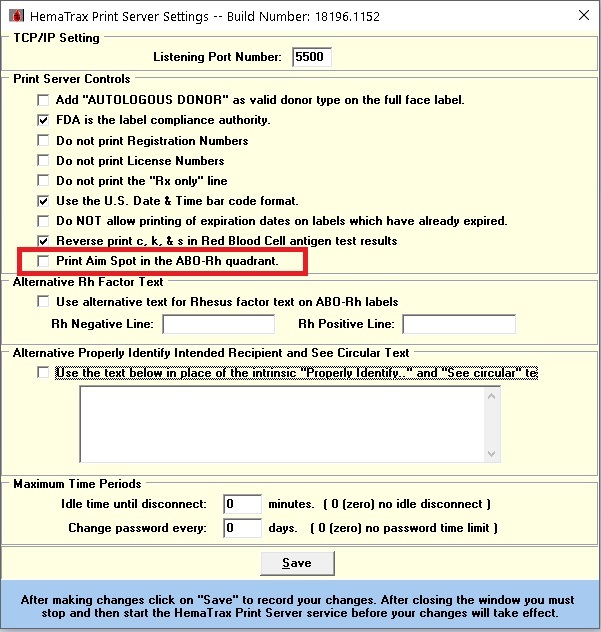
Hi Sonya, The DIAMOND symbol that prints on an ISBT128 Full Face label is a new added feature in the HemaTrax UNITY v9 (Integrated and Standalone). It's a scanning guide to decode all printed ISBT128 barcodes on a Full Face label in one trigger press. This feature, however, requires the use of a specific HemaTrax supported 2D barcode scanner -- Zebra DS8100. The feature can be easily disabled from the "HemaTrax Print Server Settings" module following the steps below (see attached picture): - Uncheck the "Print AIM Spot in the ABO-Rh quadrant." checkbox - Click "Save". - Restart the "HemaTrax Print Server" service from the Control Panel - Administrative Tools - Services. I hope the above information helps but you can always contact the Digi-Trax support team if you need additional help. Kind Regards, Digi-Trax Technical Support
-
Hematrax Software Upgrade
Hi Kathryn, Digi-Trax has a yearly paid HemaTrax maintenance subscription (LSS) and it is not associated with ICCBBA. If your facility has an LSS account and current, then you can download the HemaTrax product table update based on ICCBA's current table release (http://download.digi-trax.com) that requires your LSS login credentials. And whenever ICCBBA releases a newer product table, Digi-Trax makes sure a corresponding product table is also released. But note that HemaTrax product table update only works with UNITY v8 and v9. Kind Regards, Henry
-
Missing ISBT codes for bacterial monitored platelets (LVDS) in an open mode
Hello: Below is the information we received from ICCBBA regarding the bacterial monitored Platelets in an open system. Product Descriptions cannot contain a Bacterial Monitoring/Testing attribute in combination with any of the following: • Washed modifier • Open system attribute • Plasma reduced attribute The Bacterial Monitoring/Testing attributes are used to indicate an extension of the expiry date, whereas Washed, Open, and Plasma Reduced shorten the expiration. Any of these three would conflict with the Bacterial Monitoring/Testing attribute within the same product description. If you wash, plasma reduce, or specify the product as an open system, then the Bacterial Monitoring/Testing attribute value would need to be omitted. Thanks
-
Temperature log tag
Digi-Trax offers a 10 degree temperature indicator that our customers say is accurate, easy to use and reliable. For more information or free samples, visit: https://bit.ly/2BMtmyv or call 800-356-6126
- Convalescent Plasma
-
HEMATRAX PRINTER VALIDATION
Hello: Make sure you replace your current HemaTrax printer with the same 300 dpi print resolution and it's loaded with the Digi-Trax proprietary Blood Bank ABO-Rh firmware. Once the right replacement printer is in place and properly loaded with ISBT128 labels/ribbon (only if labels are thermal transfer), there is really no validation requirements to complete prior to its use. HemaTrax end users usually don't validate the printer, but do validate the HemaTrax software. So, as long as the printer is configured correctly (Network and in HemaTrax), then it's ready to put into use. Thanks,
-
-
ISBT 128 Product Modifications
I hope this information helps in regards to DIN on collected or pooled products. Per US Consensus Standard, the DIN should remain that of the collection facility unless the product is pooled. If the product is pooled, a unique new pool number shall be assigned by the pooling facility. This product shall be given a new Donation Identification Number (DIN) and not use a DIN from one of the units in the pool. The new DIN shall have the Facility Identification Number of the pooling facility. And below DIN information is excerpted from the US Consensus Standard v3.0.0 - 7.8.2.2. Some computer systems treat reconstituted red cells as a pooled product; others do not. The Donation Identification Number (DIN) can either be a newly assigned Pool Number (for those systems that treat the product as a pooled product) or that of the RBC (for those systems that do not treat it as a pooled product). The text name and location of the facility that appears beneath the DIN shall correspond to the Facility Identification Number within the DIN. That means, if the original DIN of the red blood cells is used, the name beneath the DIN shall correspond to the collection facility. If a new pool number is assigned to the product, the DIN shall have the Facility Identification Number of the pooling facility, and the name beneath the DIN shall be that of the pooling facility. Regardless of which method is chosen, traceability of both the red blood cells and the plasma shall be assured. The DIN of the plasma must be associated with the DIN of the final product in the facility records.
-
Irradiate label affixed to the ISBT label
We do have a label adhesive that can be directly applied to bags and meets FDA requirements. We can make a custom label if needed for an "IRRADIATED" label that can be applied to any portion of the bag.
-
Aliquot Labels
We do have a 4 x 4 ISBT128 label with proper adhesive (CFR21.sec. 175.105) available for HemaTrax that has a small back split for syringe labeling. It exposes only a portion of the adhesive and may solve your problem. The product # is ISBT-44-Syringe-23KT.
-
-
Validation plan for ISBT printer
Digi-Trax can help you with the validation script for our HemaTrax® ISBT 128 software that works with our printer. The process for validating thawed Plasma products should be the same as how these products were validated previously with your original printer. Please contact us at 800-356-6126 or at info@digi-trax.com for further assistance.
-
Unit Labels
We’re glad our labels are working for you! If you have any questions down the road, please don’t hesitate to contact us at 800-356-6126 or info@digi-trax.com
-
Irradiate label affixed to the ISBT label
For a product that is IRRADIATED, that attribute will print on an ISBT 128 label below the product name (see example) using HemaTrax software from Digi-Trax. No need for a separate label.
-
HemaTrax standalone validation
Digi-Trax can validate your stand-alone software HemaTrax; all labels. The cost for the first PC is $3000.00 and the part# is ISBT-SA. Each additional PC using the same E-Codes is part# ISBT-SA-ADD and cost is $1000.00. If you require more information or wish to talk with someone directly please call Jeff Kriozere @ 847-613-2104 or E-Mail jkriozere@digi-trax.com
-
Meditech/HemaTrax
Please call Henry Cagadas at 847-613-2114 or his cell at 847-280-4931. He certainly can help you. Henry is our main technical support person for HemaTrax.