Posts posted by Baby Banker
-
-
-
On 7/7/2023 at 6:12 PM, Mabel Adams said:
Has anyone considered how to build for cold stored platelets in their BBIS? SafeTraceTx at least, has expected temperature ranges for products on return from issue or delivery so we couldn't very well lump cold stored platelets in with other platelets. I guess we could build an entirely new product class with its own temperature range. Not that we are going to get cold-stored platelets anytime soon, much as we would love the 14-day shelf life. We are too small and remote to keep a dual inventory for oncology vs. trauma patients. I'm just curious how others plan to solve this issue. After all, the driving force of our lives is keeping our computers happy, right? Now if FDA would just approve 7-day expiration for all PR platelets!
Maybe you could make a Product Group for it. I did that with Octaplas.
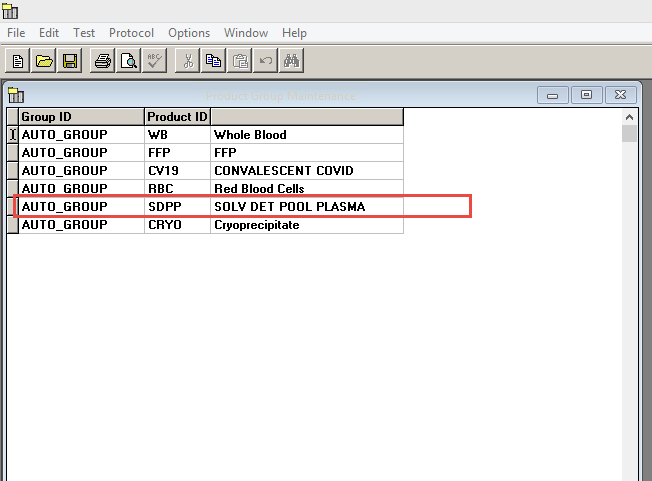
-
-
-
On 3/29/2023 at 9:41 AM, Neil Blumberg said:
Just to be clear, these regulations are almost totally arbitrary and can be overridden by a physician's judgement. There are no data to support this 30 minutes nonsense nor the 1-10 degree storage requirement. Just so we all understand there is almost no scientific or clinical basis for our regulatory rigidity and we are usually discarding perfectly safe units of blood. Rant off :).
Are there still two ranges: one for storage and another for transport? I've always thought that was ludicrous.
-
On 3/29/2023 at 8:28 AM, MAGNUM said:
yes, here in the DFW area, it only takes approximately 10 minutes or so for the internal core temp of the unit to exceed 10C. new nurses as well as old ones are still under the fallacy of the "30 minute" rule.
Aliquots will warm even quicker than that due to their smaller mass.
-
The Pediatric Trauma Society used to have some examples on their website. I'm not a member any longer, so I can' check to see if they are still there.
https://pediatrictraumasociety.org/
-
-
We send both syringes and bags through the tube system.
We have a special cap that we use for syringes for which it is obvious if someone has taken it off. That is to prevent partially used syringes from being accepted back into stock.
That was an issue for all syringes, not just the ones sent through the tube.
-
3 hours ago, exlimey said:
Thanks. Probably an unanswerable question: How low a titer is "low enough" ?
A follow-up.....can one transplant an A1 kidney into an A2 patient with anti-A1 ?
I don't know what the titer is for incompatible kidney transplant, but for hearts they prefer less than 1:4, but there are other criteria as well. If the patient is less than 12 months old, they don't worry as much about the titer.
I think they won't consider a patient who is over 2 years old.
Again though, that is for hearts.
-
-
20 hours ago, Malcolm Needs said:
This reply also applies to the excellent post above by Debbiel.
Do these "experts" not understand, as do most, if not all people involved in blood group serology (and even blood transfusion) that it has been known for years and years that not every antibody reacts by all techniques, however experienced the person performing the test may be.
I once had an anti-S that reacted by tube IAT, but refused to react by gel, even though I sent it out to a large number of hospitals who I knew used both techniques.
I also think that all true experts have either read, or are aware of Leger RM, Garratty G. Weakening or loss of antibody reactivity after prewarm technique. Transfusion 2003; 43: 1611-1614. Sadly, it would appear that (SOME) of the Quality "Experts" are not as expert as they like to think.That is, in large part, why we HAVE different methods.
-
4 hours ago, David Saikin said:
i had an AABB inspection years ago. At the summation the inspector said: "I know I'd have to dig to find something in Dave's lab." That should have been a warning. My only deficiency (which was cited by the Area Chair, who determined the deficiencies based on the Inspection report form) was that I did not have my facility ID on my antibody panel sheets. I immediately called my area chair and told him I wanted to inspect his lab (UT@Knoxville), which of course is not allowed. I became an AABB inspector/assessor after that fact.
Dr. Jones?
-
1 hour ago, Neil Blumberg said:
Another bureaucratic authoritarian idiocy? Sorry, couldn't restrain myself, but there is a cadre of "quality gurus" who are constantly thinking up irrelevant, pointless make work stuff for the rest of us. This is how civilizations come to an end. Why in the world would an SOP have to have the address, name, GPS co-ordinates, topographic elevation and postal code of the facility? How does that address any patient care issue in the universe?
I could not agree more. I believe that, if unchecked, some of the accrediting agencies will eventually regulate themselves into irrelevance.
-
You won't be cited for not being in a separate room. You can be cited if your work area is too small and cramped. We were cited by the FDA years ago. I was walking beside the FDA inspector talking to him and one of my staff walked right into me.
There was also an issue where if one of the blood refrigerator doors was open, there was no way to get from one side of the Blood Bank to the other.
Even so, Administration was not happy at all about having to give us more space. They did, but grudgingly and they gave us the least amount they could get away with.
-
On 8/3/2022 at 8:49 AM, AMcCord said:
Not a Vision user, but can you use logic in your middleware/engine to remove the unwanted characters? Is there a setting in the Vision software for how the barcode is read by the analyzer?
Unfortunately, the Vision requires the flag characters. I do not know why.
-
On 8/26/2022 at 11:26 AM, John C. Staley said:
Malcolm, my very 1st AABB inspection came about 2 months after taking the Blood Bank supervisor job. After it was over I contacted AABB and told them that I would never let that inspector in my facility again and if they tried to send her I would drop our AABB membership. YES, the inspection/inspector was really that bad and luckily I never had to carry through with my threats/promises.

I had one of those inspectors.
-
-
-
We are in the process of bringing Ortho's Vision Swift online. One of the things we would like to do is put unit confirmations on it. We've had an issue when we used the labels from the back of the units to put on the sample tubes. Apparently there is a number on the labels from the back that is different from the front label. This is causing us trouble when we try to put units on the Vision for retyping.
Has anyone encountered this and found a way around it?
-
We have SafeTrace instead of Soft, but we have made reconstituted whole blood for many years. We normally use it to prime a circuit.
We treat the reconstituted units as if it were two units in one bag. We sign both units out in the computer even though they are in one bag.
SafeTrace does not allow pooling of products which are of different product types. If Soft will allow that, you may be able to use that to avoid dealing with two unit numbers, etc.
-
-
-




Whole Blood Compatibility Testing
in Transfusion Services
Bring back minor crossmatches?