-
getting the right temperature for the cooler.
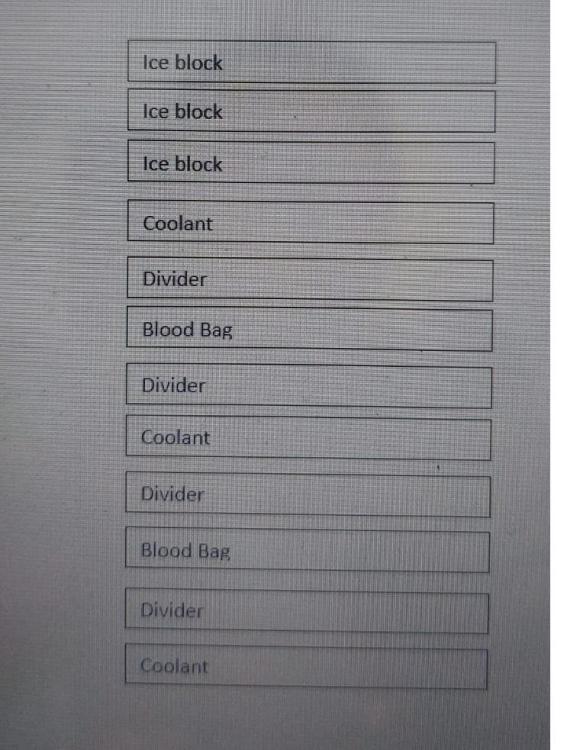
what is the configuration for all of those? so at the bottom is the coolant pack, blood bag 1, another coolant, blood bag 2, another coolant, and at the top are the three ice blocks frozen? I tried to make a picture of it, please give me your recommendation about it. how long do you prepare for it? I mean, when do you put the blood bags inside?
-
getting the right temperature for the cooler.
I am using the usual cool box, and ice pack. what kind of pre conditioning is needed? sorry, I am really don't know anything about it.
-
getting the right temperature for the cooler.
I really need some help about how to get the right temperature for the cooler. e.g. when we do cooler validation, the start was around 23-degree Celcius, we were able to reach the right temperature after 1 or two hours after we packed the Red Cells, and we only able to keep it in the right temperature for only 1 or two hours before it kept going up. how do we reach 2 - 10 degrees of Celcius before putting in the Red Cells? and how do we reach -20 degree Celcius before putting in the frozen plasma? thank you.
-
-
Key Performance Indicators (KPI)
Dear Sir, is it possible to share the 1. percentage of invalid test result 2. venipuncture failure please?
-
Look back, Trace back forms
Dear all, is it possible to share look-back and trace-back form and SOP? I'm still confused about how detail that it should be. thank you in advance.
-
QC of components
1. what to do if the QC of the components fails? 2. how to do the SPC for QC of components? do you use the percentage of failure or the number, e.g. hemolysis rate.
-
Helmer Freezer Validation
dear Mam, could you please share more with me? really would like to hear it from you.
-
Freezer out of Temp
question: is there a certain temperature and time as a limit before we way it has to be thrown away?
-
Freezer -30C Thermometer uses sand instead of glyerol in bottle
dear Sir, what kind of sand? is it the sand for the cat or any sand will do?
-
-
-
Blood Component Making Validation Process
thank you
-
Novel Corona Virus
as far as I know, it's a model from a computer...
-
Hi again
Hi again, I forgot whether I have introduced myself before, but I will just think about it as my first introduction. I'm Nova from Indonesia. Before I worked as a doctor in Hospital Transfusion Services so I asked a lot about blood transfusion reactions and blood administration. Not only in this forum, but also in other places, like ISBT congress, etc. But at the moment, I work for a Blood Transfusion Unit (where we do blood collection and blood processing). and we are in the middle of improving the quality, like implementing GMP, and as part of GMP do validation qualification. So I may ask more questions almost about anything. Thank you or in my country, Terima Kasih... Nova
-
Blood Component Making Validation Process
Dear all, I have to do validation process for the blood component making for each components that we made. But so many things still made me confused. 1. How many blood bags should be used for validation process? Is there any rules of how to calculate it? 2. Is it possible to do it more than one times? e.g. when I need to validate 60 blood bags, can we do it in three parts, each 20 blood bags? I asked the second question because in my country, the auditor said that we have to do it three times (because according to Pharmaceutical GMP, we have to do it three times). But when I ask about how many and how, it just answered by there is no certain rule about it. 3. Do we have to do it three times? e.g. when I need to validate 60 blood bags, do we have to do 3 x 60 blood bags instead of 1 x 60 blood bags or like question number two, 3 x 20 blood bags? So, how do you do it in your place? Thank you.
-
PRC and FFP component making process validation
my BTU is trying to do the validation and qualification program. one of the GMP auditor asked for the validation process for component making (focusing on RBC and FFP first). is there any example that I can use as a guide? thank you in advance.
-
Pre Medication
how about moving to my country, at least they still do it because they don't know... (trying to build a high hope, that they will change after they know)...