Everything posted by RRay
-
Weak Backtype Resolution
Working off of hand-me-down blood bank folklore is a habit I'm having a hard time breaking with my techs. Pray for me. Inversely, I've never had the 4 drops procedure in place at any place I've worked. I follow your logic, but I can't find any recommendation of the 4 drop practice in the Technical standards or methods, or anywhere. I often wonder how certain practices got started.
-
Documentation/Testing for additional samples needed.
I think you misunderstood. I'm asking about when you run out of sample when doing complex testing. How do you document the additional samples to complete the workup. Are you saying you wouldn't complete the workup unless Hb was low? How were you able to record them as spare?
-
Weak Backtype Resolution
I'm fairly new to my current institution but noticed some techs are resolving weak backtypes by adding 4 drops of plasma to the A1/B cells and documenting such. However, this is not an allowance in our policies anywhere. I have a faint memory of this being addressed somewhere in the past about not doing this because if you have to add 4 drops of plasma to detect the AB antibodies, you should do the same in your ISXM and that is very hard to track when you need to do so. Currently the SOP only allows for an extended RT incubation and a 4C incubation to resolve these missing reactions. Does anyone have any feedback on this? Is the plasma being weak an issue for the ISXM? I mean, that is the whole point... to detect ABO discrepancy. We are stuck with no electronic XM until mid-late next year.
-
Anti-G????
I've had this come up before with incomplete transfusion history (transfusions at another facility) and with IV drug use / sharing needles. Occam's razor?
-
Documentation/Testing for additional samples needed.
Anyone else care to share their protocol for documenting additional specimens needed for workup?
-
Record Retention for Shipping Documents
This is facility specific, no reg. I confirmed this with supplier as well. My predecessor kept for 10 yrs. I added it to the retention policy as 1 yr plus current. Was able to trash boxes worth.
-
Documentation/Testing for additional samples needed.
Thanks for your feedback! I'm surveying the audience to see what can be pieced together as most efficient. Currently, my LIS requires override when a specimen being used for XM has not had a T&S on it. This LIS is going away next year tho. I have only used blood bands at my current institution and am evaluating getting rid of them. They cause a scary % of redraws (nurses cutting the wrong band, or rebanding when transferring between units, etc) and delay in patient care when patient ID isn't at question. And they're expensive.
-
Documentation/Testing for additional samples needed.
This process has been different every place I have worked. How do you handle documentation and use of additional samples when needed to complete a workup? Do you ABO/Rh type the new sample? T&S? No additional testing? Does your LIS support this documentation? I'm not aware of any specific standard that says the sample you use to XM has a T&S performed on it.
-
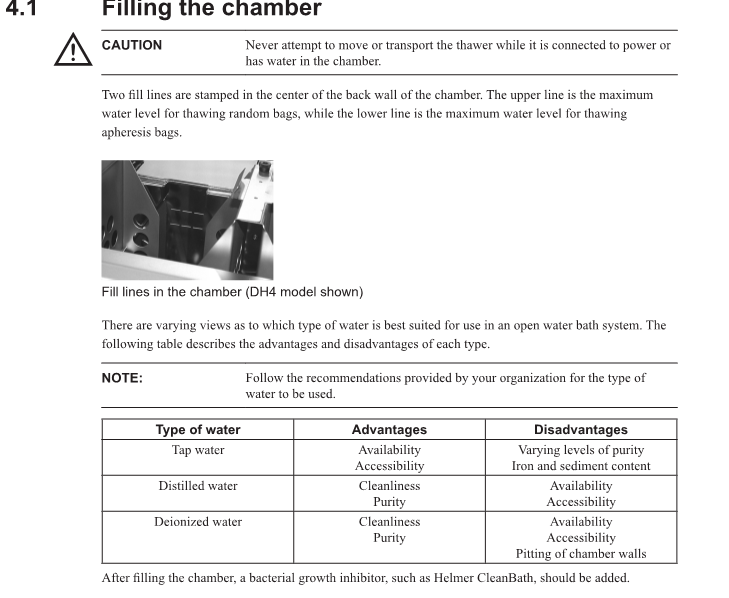
Cleaning the Helmer Plasma Thawer
I think this depends on the version you're dealing with. I've used DI after there was a problem getting distilled water at my current facility and one of my previous jobs used DI for 10 years and it never had deposits or issue. BUT, I worked at a place briefly that used tap water and it was the nastiest plasma thawers I've seen, even with weekly cleaning and CleanBath addition. My helmer model maintenance manual says this:
-
Staffing!
When I was moving to the area I couldn't find ANYTHING blood bank specific. That's why I'm in Cheyenne. Sad to hear there's such a vacancy increase. The lab here is on the upswing. Having trouble finding generalists for nights, but we've had luck with H1B visa sponsorship in the past and are considering that again.
-
Staffing!
We only allow the non-lab-educated with bachelor's degree to work as micro techs with the expectation that they will sit for the categorical after time is put in. I'll work the bench every day before I allow that in blood bank. Like Ensis said, you'd have to teach them everything. Blood banking isn't touched upon in any biology or chem degree... or any degree besides lab science that I know of. It's hard to instill critical thinking on a topic they don't have the theory for. It would just be following IFTTT process, in best case scenario. I mentioned in another thread, the problem we are facing in addition to the staffing is that we have very few people who are eligible to assess competency. For CLIA, must be at least be a bachelors with 1 yr experience for mod complexity assessment. We have a lot of MLTs or brand new MLS techs.
-
Max LowT WB units timeframe post-MTP
Just to follow up, this is what we decided with the medical director. Max 4 LowT Group O WB per MTP event. If pt types and confirms as Opos there is no max (inventory would be the limiting factor here). I think this is the meet in the middle with meeting standards and quickness in issue/infusion. It is also an amalgam of previous employer SOPs and other facilities in the area.
-
Rural area lab staffing
We allow non-lab degrees for some micro positions only, with the intent they can sit for the micro categorical exam. We also use H1B Visa sponsorship programs. That's a couple things we've done to help. The problem we're running into is that with CLIA competency requirements, we don't have very many people who are qualified to evaluate competency. We have a lot of MLTs and new MLSs. The MLTs can't assess mod. complexity tests and the MLS need 1yr of experience performing the test to evaluate. Blood bank is the most intensive competency to do, so having at least one evaluator per shift is ideal. I'm a BB sup in a small regional med center.
-
Ortho Panel A and B quality control
A lot of the standards list "Or as recommended by the manufacturer." CAP for example. This is usually my out, as manufacturer insert does not suggest reagent reds be QC'd. They are quality controlled by the manufacturer as part of the FDA compliance.
-
Grifols analyzer users: Roll call!
I have experience with Grifols analyzers but am currently with Ortho. With the multitude of supply issues and delayed shippings I'm trying to see if Grifols is feasible, especially in my area. I don't know of anyone in the West-Midwest using them. Everyone I know on Grifols is east coast or west coast. Curious to know if you've experienced any supply delays, back orders, etc. The core lab is shopping for alternatives to Ortho, so I may as well too. Any feedback appreciated!
-
Dream equipment/products/supplies?
Yes! And if your LIS is compatible it can even EXM it for you if testing is complete. Blood kiosks are on my list, but I dunno if this grant can handle that price.
-
Dream equipment/products/supplies?
The temp monitoring is crucial. I don't know if I could go back. If I didn't have it, that's what I'd get.... although I'd choose a different company. I'm not sure if RFID would be beneficial for a small hospital like mine. Maybe for coolers but we even have less than 10 of those.
-
BloodBankTalk: Correct Blood Bank Nomenclature
I just answered this question. My Score FAIL
-
Sunquest Capabilities vs SoftBank Capabilities
I left Sunquest in 2020, with the latest update. They had minimal transfusion reaction documentation that we had to supplement with EHR print outs. No on board QC. They did have an inventory search and paired with SmartTerm can print inventory reports as built. As a blood bank we were going paperless, so all of the QC and maintenance documentation and such was done through excel spreadsheets.
-
Unit confirmation on the Vision
I believe our middleware does the conversion.. We're on an older safetrace version. The only issue we have with donor confirmations is when you get multiple containers per unit #. They have to be manually programmed or done by hand and entered into BBLIS.
-
Dream equipment/products/supplies?
Space... the final frontier. More space would be excellent! For us, 2 people in there is too many... add a student or trainee in the mix and it's uncomfortable.
-
Dream equipment/products/supplies?
I wish. Unfortunately the grant can't be used to supplement wages or add FTE.
-
Ortho Vision LIS Sunquets
Are you by chance using smart term with sunquest? Several years ago I had this configuration and we had to use smart term to "flush" the middleware.
-
Max LowT WB units timeframe post-MTP
That standard is addressed here as the 4 max WB and as outlined within MTP and emergency release policy. A previous facility I worked at used WB for MTP until they ran out or were able to complete the Type and screen +ABOconf. Then XM PRBCs/FFP/PLT/CRYO rounds. The time frame there was per MTP, with unknown blood type. Tricky thing at my current facility is that WB is first two rounds of MTP regardless of current testing or blood type, so theoretically they could qualify for WB again with a new MTP activation, or under a new admission per se. Maybe I'm thinking about this too hard, but the SOP I'm working with seems a little thin and hasty. Why give uncrossed Opos WB to a patient you know is Apos (current T&S) just because they're initiating MTP? Only thing I can figure is that it's quicker to issue 2-4 units of WB versus a 4/4/1/ or a 6/6/1 MTP round.
-
Blood components for Patients with positive antibodies
I haven't seen a filter but I've used retic harvest technique to "filter out" non-native cells for antigen typing when someone has been transfused in the past 90 days. Is this what you mean?