Search the Community
Showing results for tags 'CAP'.
Found 10 results
-
API AFB Survey
We just had an API AFB survey where the specimen was put on the back of the slide... Not the labeled side. After the staining process, the specimen circle didn’t take on any color and nothing would come in focus. I stained the other side and sure enough, positive!!!! API’s response, is we should notice where the specimen is regardless of the labeling!
-
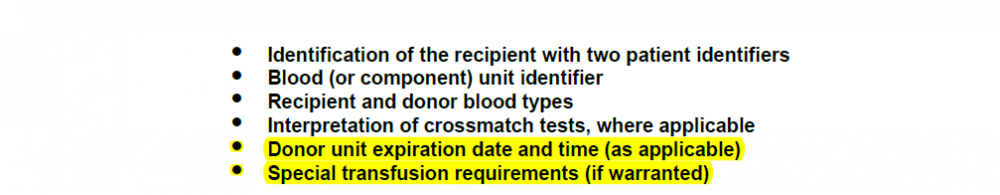
CAP TRM.41350
Per the CAP requirement, highlighted items are added. We are using very small label with SOFTBANK which has minimum requirement. (first four items) How is everyone complying with below requirement. 1) Are you changing your label size? Thanks
-
Computer Crossmatches and Revised CAP regs
The 2017 revisions were released this week. The computer crossmatch section was revised to align with the FDA guidance. "If ABO typing discrepancies exist, you should not rely on a computer crossmatch.This is particularly important if there is mixed field red cell reactivity, missing serum reactivity, or apparent change in blood type following hematopoietic stem cell transplantation.Under those circumstances, your procedures should provide for compatibility testing using serologic crossmatch techniques." I called CAP to verify that this included resolved ABO discrepancies. Our hospital had been allowing computer crossmatches for ABO discrepancies that are resolved, and I'm not sure that our BB LIS could prevent a patient with a resolved ABO discrepancy from receiving a computer crossmatch. Does your LIS prevent computer crossmatches for patients with MF reactions or weak reverses?
-
external alarm monitoring of freezer and refrigerator
Do the Blood Bank refrigerator and freezer have to be externally monitored outside of the blood bank? The lab is staffed 24/7 and able to respond to the freezer or refrigerator alarm. Does security or the Emergency Department have to also externally monitor these alarms? Are there any CAP or AABB standards, or does the FDA require this? Just wondering since it seems to be redundant, and we have an older freezer which may be difficult to hook up.
-
CAP TRM.40120
I need some guidance! I did a quick search of the forums for any discussion about this, and the most current posting was in 2010. I'm wondering if anyone has any new information, new experiences, or any advice about CAP TRM.40120. The note states "...all analysts participate in QC on a regular basis." How frequently is "regular"? For example, when I was looking to complete our annual Competency assessment in September (don't ask...), I was looking for evidence that each individual who performs MTS testing had performed MTS QC. There were some employees who had not performed MTS QC yet in 2016. I'm inclined to say that someone who hasn't performed QC in at least 8+ months is not participating in QC on a regular basis. Being new to my role, I'm just not sure how the assessors interpret this standard, and how others provide evidence of compliance.
-
Eye protection requirements (GEN.74100 & 74200)
My interpretation of these two standards is that eye protection must be made available and instruction on proper use must be given to all the techs. But not that goggles/glasses must be worn at all times in a clinical laboratory regardless of the risk of splash/spatter/aerosol formation. Do your facilities require that techs wear eye protection at all times or only when performing tasks that have a high risk for exposure? Thanks! GEN.74100: Appropriate personal protective equipment (gloves, gowns, masks and eye protectors, etc.) is provided and maintained in a sanitary and reliable condition in all technical work areas in which blood and body substances are handled and in circumstances during which exposure is likely to occur. GEN.74200: Personnel are instructed in the proper use of personal protective clothing/equipment (e.g. gloves, gowns, masks, eye protectors, footwear) and records are maintained.
- CAP competency requirements
-
GEN.55500 CAP Competency Assessment
Can anyone provide some help/insight into how they identify each "Test System" to satisfy the 6 elements of competency for CAP? Personally, I feel like "Blood Bank" should be it's own "test system" and I should be able to assign all the different testing we do into one of the 6 elements based on the errors/problems I see throughout the year. Then rotate all the different testing systems, using the 6 elements, annually. it specifically says "if there are any tests with unique aspects, problems, or procedures within the same testing platform competency must be assessed as a separate test system system to ensure staff are performing those aspects correctly" Am I really supposed to perform all six elements for: ABORH/ABS using echo, gel, tube XM using gel, tube Antibody Identification using gel, tube, ficin, diluting 3% down to 08% for gel testing, etc,etc,etc Dat using Gel, tube Just using the above examples that's documenting 66 elements annually and that's only scratching the surface! How many "test systems" have been able to narrow your blood banking down to? I feel like I need to hire someone just to do competency assessments!
- AABB deemed status for CAP?
-
Proficiency testing for automated DAT
Our facility has decided to start running IgG DATs on our echo (for cords and investigation of warm autos) but there is one snag in the road - neither CAP or API offer an Automated DAT survey at this time - so what do other facilities do? We have a couple ideas but would love to hear what others are doing to stay proficient if running DATs on any automation... 1. Old school blind comparison? - procure blind samples from another facility near by that performs DATs on automation 2. Use the samples from the J (manual) CAP survey on the instrument - but how to report it out on the survey??? 3. Other suggestions????