Dansket
Members - Bounced Email
-
Joined
-
Last visited
-
Country
United States
Everything posted by Dansket
-
Using only mixed field anti-D (ortho gel) as qualitative indicator for RhIg eligibility
I'm not disagreeing that other types of serological phenomena are seen as mixed-field reactions when viewed microscopically on a slide, but can only speculate as to how these reactions are observed in gel. I've seen A3 and anti-Sda mixed-field reactions through a microscope on a slide but not yet in gel. I'm curious, have you see an A3 in gel?
-
Using only mixed field anti-D (ortho gel) as qualitative indicator for RhIg eligibility
Malcolm, please re-read my post. Mixed-field is a term used to describe the observation of a microscopic reading on a slide of the contents of a standard tube test where there may be seen "individual clumps of red cells in sea of unagglutinated red cells". Two populations of red cells will be present in a group A recipient of a group O red blood cell transfusion. When testing a post-transfusion blood sample, a band of agglutinated red cells will be trapped at the top of the gel column, a button of red cells will be seen at the bottom of the gel column and no red cells trapped within the anti-A gel column. These observations are visible without the aid of a magnifying device and are graded as dp dual-population according to MTS-ID Gel Card Interpretation Guide used for both manual and automated gel testing.
-
Using only mixed field anti-D (ortho gel) as qualitative indicator for RhIg eligibility
There is no such thing as mixed-field in gel testing. The term mixed-field is used to describe microscopic reading of tube tests. Gel tests are read macroscopically and use the term 'dual-population'.
-
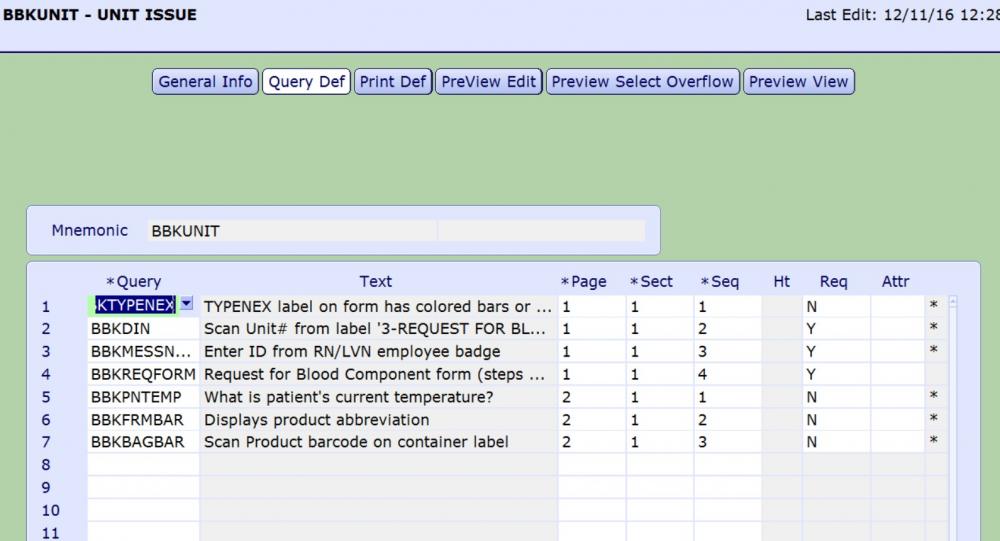
Meditect ISBT card
There is a dictionary titled MIS Customer Defined Screens. Attributes are written in Magic Programming code and that is what you see above. Let me know if the attached file helps. QUERY BLOOD ISSUE.docx
-
Meditect ISBT card
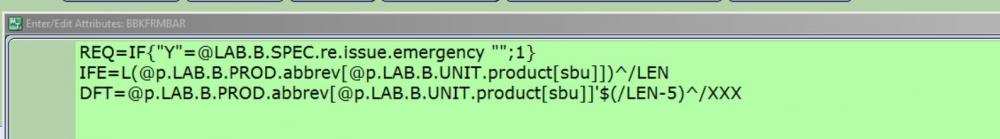
My strategy to confirm the correct product is to add queries to the blood issue by patient routine. This attribute reads the product abbreviation of the component selected for issue and defaults the result into the BBKFRMBAR query. This query requires user to scan Ecode on the blood component container label, verifies that ISBT128 barcode was scanned and then compares Ecode scanned into BBKBAGBAR to Ecode from BBKFRMBAR.
-
Meditect ISBT card
-
SIGNIFICANT ANTIBODIES FOR ELECTRONIC XM
Darren, Put it a service request for custom keyword. I have had some success with this approach in the past. I have intentionally not trained staff to add or remove markers. If a positive antibody screen entry is saved and verified, a whole lot of tests are automatically reflexed. So it is much more than just correcting a marker. I review all antibody identification workups for appropriate computer entry. It hasn't been an issue ....Yet.
-
SIGNIFICANT ANTIBODIES FOR ELECTRONIC XM
Correct, the antibody markers are stored in MRI History. There are many other markers in BBK History and I only wanted antibody markers to print on the Meditech specimen collection labels because of limited space on the label. We are using Meditech C/S 5.67. We don't enter antibody identification panel results into Meditech. Per Iatric, there is no way to cause antibody markers to be added when the test ABID is resulted using the BBK Antibody dictionary.
-
D typing
Currently, to avoid any confusion amongst physicians, nurses and generalist blood bank staff, all patients are reported as either Rh Positive or Rh Negative. Meditech is configured to automatically reflex Weak D test and Weak D control on newborns whose red cells do not react with anti-D in the A/B/D Monoclonal and Reverse Grouping Gel card on ProVue. If the Weak D test is agglutinated and the Weak D control is not agglutinated, again on ProVue, newborns are classified and reported as Rh Positive without any disclaimer. BTW, I thought the term "Du" was obsolete and had been replaced by "Weak D".
-
Anyone using the Vision (new Ortho analyzer)?
Does VISION allow for running selected Panel cells or a Room Temperature panel?
-
RHOGAM "ANTIBODIES"
If current antibody screen is negative, these patients qualify for electronic crossmatch in our facility.
-
SIGNIFICANT ANTIBODIES FOR ELECTRONIC XM
So does Meditech C/S 5.67. For security purposes, our IT department has configured all terminals to automatically log out after 5 minutes of inactivity. For our purposes, it is extremely efficient to be able to visually access that information from the specimen container label as opposed to the many computer steps required to access the information from the Meditech; login to terminal, launch Meditech, login to Meditech, navigate menu system to Enter/Edit results, scan in specimen number, click on tab to view history, select history option and finally view antibody, etc.
-
SIGNIFICANT ANTIBODIES FOR ELECTRONIC XM
The markers are configured as MRI History markers. See attached file The ABID marker is added automatically whenever an antibody screen is resulted as positive. The antibody marker is also added by a calculation, but it was written by a third party "http://new.iatric.com/" (it has to be triggered by a test that is always resulted, but can't be done by directly by resulting your ABID test). markerdict.docx
-
SIGNIFICANT ANTIBODIES FOR ELECTRONIC XM
You're almost there. My "ABID" = your "AbHx". I created markers for each antibody specificity, using the same keyword "bsp mri mark".
-
Grifols Erytra
ORTHO ProVue also uses bleach to disinfect the lines.
-
pre-transfusion sample aliquot
To me, it is not a common practice, because I eliminated it wherever I encountered this practice. Separating serum/plasma for a Reference lab is a separate issue because you are sending them a known problem. Do you have an example of a problem that occurred in your facility that was directly linked to not separating serum/plasma from a whole blood sample immediately after centrifugation? Did this problem occur in a known patient or a patient new to your facility? Does this occur frequently, rarely or is this the only known example? These are some of the questions I would ask before memorializing the process in a procedure manual. Unless you have accumulated evidence to support the assertion that separating serum/plasma actually prevents problems frequently encountered in your facility, you have to look to other reasons to justify doing this. If this problem occurs in 1/10 patients, I would be doing it. Otherwise, I view separation of serum/plasma from red cells to be a “solution looking for a problem”.
-
pre-transfusion sample aliquot
Why do you aliquot these blood samples? Do I assume correctly that you are separating serum/plasma from red cells? I am adamantly opposed to routinely aliquotting pre-transfusion blood samples for several reasons. It is an unnecessary step that does not enhance the process. It iintroduces another potential point of failure. It takes time. Storage space requirements are effectively doubled.
-
SIGNIFICANT ANTIBODIES FOR ELECTRONIC XM
Upon completion of initial testing with a conclusion of INC, samples are routinely sent to a reference laboratory. If the reference laboratory concurs with our result, we enter INC in Meditech. If the current antibody screen is negative and there is a historical antibody identification reported as INC, the patient qualifies for electronic crossmatch.
-
SIGNIFICANT ANTIBODIES FOR ELECTRONIC XM
For specimen requirements, do you require two ABO specimens drawn at separate times (could use a Heme specimen or even coag??) a retype of a sample by two different technologists or retype by same tech if sample drawn using a "mechanical barrier system or digital bedside patient identification system" (per CAP checklist), for which we have Mobilab. We don't have a "mechanical barrier system or digital bedside patient identification system". For patients without a historical ABO on file, we test the uncentrifuged blood sample with Anti-A,B antiserum. That test result is entered and filed in Meditech. If the anti-A,B test is agglutinated, then Meditech reflexes the test CONFIRM which is ordered on a new specimen number that requires a second venipuncture. Our BBK specimen collection labels are configured to view historical ABO on file and antibody identification on file without login and search in Meditech.
-
SIGNIFICANT ANTIBODIES FOR ELECTRONIC XM
NAD = A positive antibody screen and no agglutination observed in panel (all our testing is done in Gel on ProVue) INC = A positive antibody screen and some but not all panel cells agglutinated with no apparent specificity
-
SIGNIFICANT ANTIBODIES FOR ELECTRONIC XM
I agree with your antibody list. Generally speaking, patient's with a negative antibody screen meet criteria for electronic crossmatch, excepting patient's with a history of clinically significant antibody. Our policy states that if the patient's current antibody screen is positive, do serologic crossmatches (no exception). If the current antibody screen is negative and the patient has a history of anti-Lea, -Leb, -P1, -M, -N, COLD, WARM (no specificity), NAD, INC, -RhiG, that patient qualifies for electronic crossmatch. All other antibody specificities are considered clinically significant and require serologic crossmatching regardless of the current antibody screen test results. Your future policy for electronic crossmatch could mirror your existing policy for immediate-spin crossmatch only. Then I challenged you to review your facility's data to determine how many cases, if any, did serologic crossmatching detect a clinically significant antibody in a patient with a negative antibody screen.
-
SIGNIFICANT ANTIBODIES FOR ELECTRONIC XM
Do you have a way of listing patients from your system who had incompatible crossmatches with antigen-negative units antigen-untyped units in the presence of a negative antibody screen? Are you asking about patients whose current antibody screen is negative but have a history of antibody? If so, the following antibodies are configured as clinically insignificant in our Meditech system when then allows electronic crossmatch: -Lea, -Leb, -M, -N, -P1, COLD, WARM, NAD, INC, -RhiG. In response to Malcom and on an individual basis, you could create an anti-M (M37) that is clinically significant and an anti-M (M) that is clinically insignificant.
-
Can't access ICCBBA website
Does anyone get this message when attempting to access ICCBBA.ORG website? I've tried many times over the past 3 days without success. Internal Server Error The server encountered an internal error or misconfiguration and was unable to complete your request. Please contact the server administrator, you@example.com and inform them of the time the error occurred, and anything you might have done that may have caused the error. More information about this error may be available in the server error log.
-
Provue vs Wadiana
Darren, ORTHO reagents and gel cards don't work on Wadiana.
-
Meditech Emergency Issue & Electronic Crossmatches
Not yet (I originally submitted request in 2011)! Development prioritizes such requests. My request is on the bottom of the list because there are few users of both Electronic Crossmatch and Emergency Release. So if you are interested, please BUG THEM!