-
-
-
Rh antigen typing on Vision
Why not just use the correct reagent thats inteded for the Vision?
-
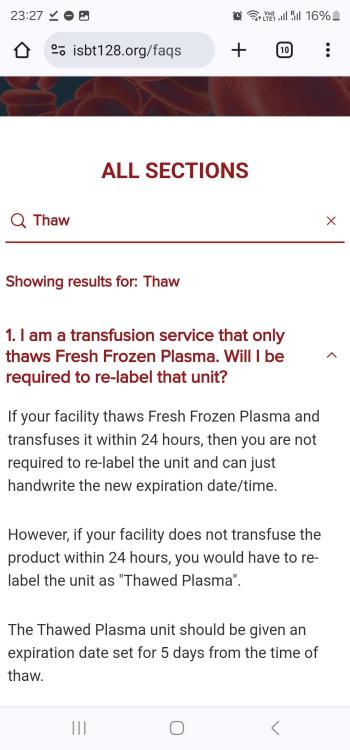
Thawed FFP
-
Thawed FFP
Did the advise in the attached screenshot from this site change? And if so which standard, document or reference etc details this requirement?
-
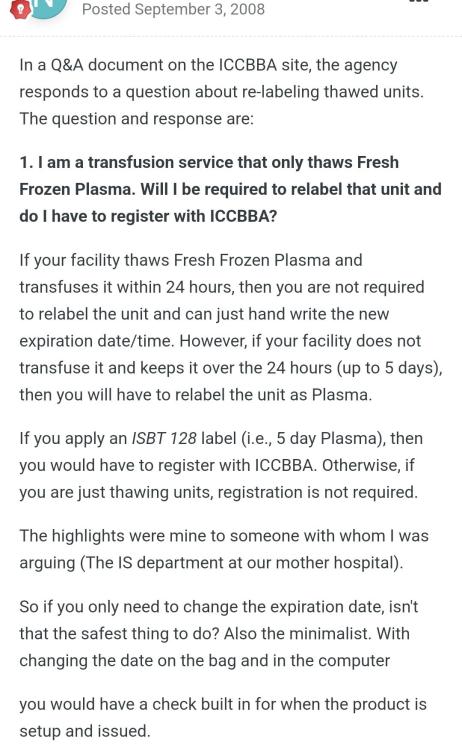
Thawed FFP
-
Thawed FFP
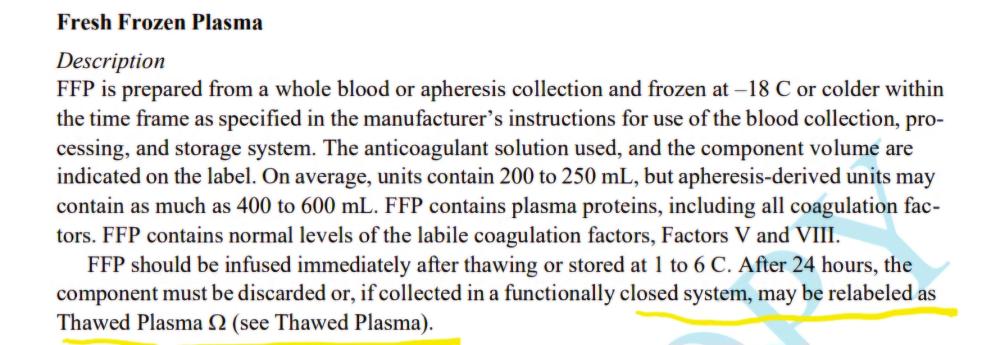
AABB standard as shown in the screen shot mentions if issuing as FFP(after thawing), this implies it is ok to issue as ffp without updating the product? Is there any standard that states it should be relabelled?
-
Thawed FFP
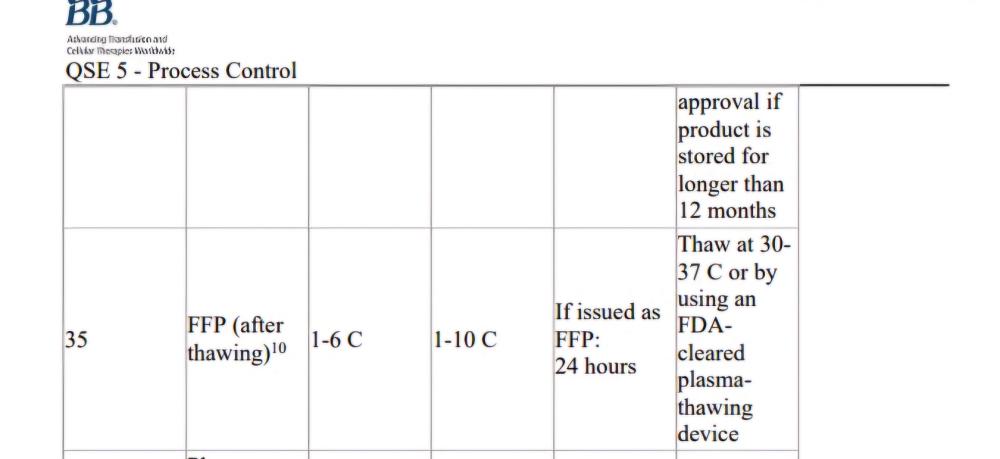
Is it acceptable by both CAP and AABB standards, to use FFP after thawing without changing the product code on the label to "Thawed FFP", only updating the expiry? (Up to 24 hours post thaw) TIA
-
-
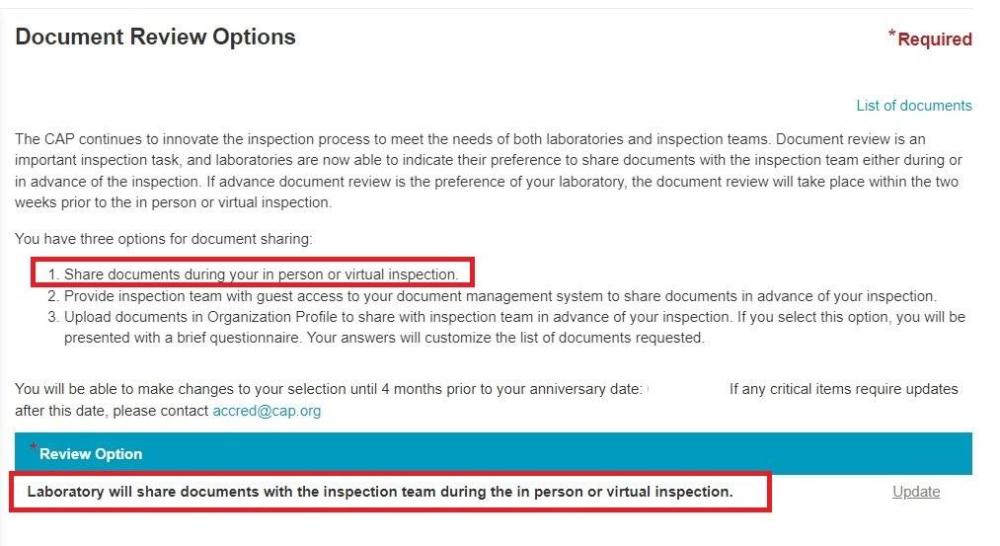
CAP Inspector Requesting Documents in Advance
They requested by e-mail and haven't sent any official sharing links so I guess there expecting them back by e-mail which from what I've found from the CAP website is not one of the acceptable ways of sharing documents in advance., and yes, the list is 3 sides of A4 paper long so its a pretty large list. I did find the below on the CAP website, and it seems there is an option to "Opt" for advanced document review, however we have not, so I think we will be declining this. Thanks
-
CAP Inspector Requesting Documents in Advance
We have an upcoming CAP inspection for all Lab sections and today we have received a big list of documents, Policies and Procedures etc, that one of the inspectors wants us to send them in advance. This is the first time we have experienced this with CAP especially as its an onsite, not remote inspection. Thoughts on this?
-
Temperature Alarm Testing
We have an nonconformance for temperature alarm activation testing, as per the assessor they wanted to see the alarm activation at exactly the set alarm temperature. I feel this is unrealistic to get it exactly on the set activation temperature. Is there any references or guidance on this I can follow for what is required? I've looked in the AABB Technical manual but don't seem to be able to find anything specific, what I'm looking for is some guidance on how close to the stated alarm activation temperature, the alarm must activate. Thanks
-
Making Own Kleihauer Reagents
In our part of the world we are unable to purchase any commercially available Kleihauer reagents/kits, we have started making our own from the base chemicals following the method described in the AABB test method guide. I'm assuming this would constitute a LDT under CAP? in this case for validation and QC purposes what would we have to do in addition to the usual test method validation and QC's with every batch? if anything. Just to clarify, we are making up the Citrate phosphate buffer and the Eosin solution, the Hematoxylin is a commercially supplied. TIA
-
-
Chido / Rodgers Identification
Chido and Rodgers can be neutralized with Chido/Rodgers positive plasma, what are the antibody characteristics that suggest such an antibody that make you decide to do the antibody neutralization? My understanding is they will be pan-reactive with most if not all identification panel cells and non reactive in enzyme, are there any other characteristics that should lead to the decision to try neutralization or should it be performed on any pan-reactive, non enzyme reactive AB? Thanks
-
-
Kleihauer sample timings
5.3 Timing of samples The maternal sample for FMH estimation should be taken when sufficient time has elapsed to allow fetal cells to be distributed within the maternal circulation following delivery, manual removal of placenta or sensitising event. A period of 30-45 minutes is considered adequate (BCSH 2006a). BCSH FMH Guidelines 2009
-
-
-
Barrier method
We have a blood transfusion administration module in our HIS, on receipt on the ward they have to scan the unit against the patients electronic record, if it doesn't match the patient it was issued for, it will not allow them to proceed with the administration. I am working to further enhance this by incorporating patient wrist band scan at the same time at the patients bed side.
-
Rosette Test Reagents
I want to introduce the Rosette test for FMH screening, unfortunately no commercial kits are available in the UAE where I am based. Right now I cannot even find a Keilhauer staining kit. Does any one use an in house method for this test? if so what Anti-D reagent do you use? AABB have a method which just states high protein Anti-D reagent, can anyone offer any further advice on selecting a suitable Anti-D antisera for use with this method? Thanks
-
 Malcolm Needs reacted to a post in a topic:
Retention of records for patients with passive Anti-D injection
Malcolm Needs reacted to a post in a topic:
Retention of records for patients with passive Anti-D injection
-
Hemolysin Titres
I am looking for a method for performing Hemolysin Titres on blood components, does anyone have one they would be willing to share? Thanks