Posts posted by Dansket
-
-
-
3 hours ago, Malcolm Needs said:
I must admit to being a little confused by your first sentence, "The user (not the computer) in my facility selects a RBC component from the refrigerator for crossmatching.", as in "The user (not the computer) in my facility selects a RBC component from the refrigerator for crossmatching." there is human intervention by the "selection" by the human, and not the computer, means that it is not, by definition, either a "computer crossmatch" or a "computer issue"? I am quite prepared to be persuaded otherwise.
Some computer systems allow user to select the component from a list displayed on screen, very, very risky in my opinion. My approach requires user to select component from refrigerator and scan the barcoded DIN into the BBK Enter/Edit results routine. These same steps are done, whether a serologic or electronic crossmatch is indicated. The only difference is that a donor tubing segment must be removed from the blood container for a serologic crossmatch.
Based on my reading of your reply, do I understand correctly that you have not personally configured or operated a computer system qualified for the computer crossmatch in a Transfusion Service?
Prior to any "crossmatching" in a computer system, user must enter results of ABO, Rh and Antibody Detection testing on the patient and ABO/Rh confirmation testing on the rbcs from a donor tubing segment. This means results of anti-A, anti-B, anti-D, Rh control, A1 cells and B cells for the patient and anti-A, anti-B, anti-D, and anti-A,B (however it is done) on the donor rbcs are entered into the BBK Enter/Edit results routine. The user entered results are compared to "truth tables" and ABO/Rh interpretations are generated accordingly.
It is simple process for the computer to "match" both patient and donor unit when both are ABO/Rh identical. It is more complicated for the computer to "crossmatch" when the ABO/Rh of patient and donor unit is not identical. "Crossmatching" is accomplished by another "truth table" that is configured by the user to allow selection of ABO/Rh compatible but not identical donor units for a patient.
In my system, if a patient qualifies for a "computer crossmatch", the system automatically enters 'NP' as the results of the immediate-spin and IgG crossmatches. If a patient does not qualify for a "computer crossmatch", user must enter serological test results for the immediate-spin and IgG crossmatches.
BTW, I believe your dates for article by Boral and Henry are incorrect. Their article was published in the journal Transfusion in 1984 and the AABB approved the concept of the Type and Screen in 1987.
-
Edited by Dansket
changed wording2 hours ago, Malcolm Needs said:But in what way, EXACTLY, is that a crossmatch? Certainly the computer's logic programmes are "selecting" a unit, but they are not performing a crossmatch. This is why a serological crossmatch is required if a clinically significant antibody (other than ABO antibodies) are present (well, that and the fact that once an antibody has been identified, there is every chance that there are other specificities, often directed against low prevalence antigens, present in the plasma).
The user (not the computer) in my facility selects a RBC component from the refrigerator for crossmatching.
Are you restricting the definition of a crossmatch to include only serological methods? In 1987 the AABB allowed users to limit the serological crossmatch to the immediate-spin test if the antiglobulin antibody screen test was negative. Then, in 199?, the AABB allowed users to replace the immediate-spin crossmatch with a "computer crossmatch" to detect ABO incompatibility. Before this, the word "crossmatch" was prefaced with "major" and "minor". So the definition of "crossmatch" has undergone many changes over the years.
The FDA allows Transfusion Services to use a qualified computer system to detect ABO incompatibility between donor and recipient in lieu of serological methods to detect ABO incompatibility, i.e., as stated by tricore, "Computerized Analysis of the Compatibility between the Donor’s Cell Type and the Recipient’s Serum or Plasma Type".
-
2 hours ago, Malcolm Needs said:
I am not arguing that the FDA call it a computer crossmatch, indeed, I am sure they do. However, what I am asking is, where in the entire procedure does a crossmatch, particularly a crossmatch performed by a computer, take place? The answer is, it doesn't!
In the Meditech computer ecosystem, the crossmatch occurs when the user enters donor unit numbers into the BBK Enter/Edit results routine. Blood components are released for transfusion using the BBK Issue Units routine which is separate and distinct from the BBK Enter/Edit results routine.
-
-
22 hours ago, NewBBSup said:
We will be implementing computer crossmatching this spring. I'm starting to write the procedure for this. Would anyone be willing to share their procedure with me? I'm having a problem coming up with something intelligent for the purpose section.
I also have seen in previous feeds that most of you do NOT require an ABO/Rh verification on the sample if another tech wants to set up units for a computer crossmatch. Our current policy now is if another tech crossmatches on a sample that someone else did the type and screen on they must also type the patient's sample for verification. I'm hoping I can eliminate this for speed and ease since the computer crossmatch is supposed to be quicker.
Which computer system are you using? Are these "type the patient's sample for verification" being recorded in your current computer system?
-
4 hours ago, Gkloc said:
If you are using the ALBAQ controls we use sample 4 and spin it down and remove the supernatant (or most of it) and add 4 or 5 drops of anti-D to it then re-centrifuge. This will give you a positive result for controls. I am currently in the process of validating the Vision and performing correlations, but this is what we use for QC on the ProVue.
We mix Alba-QCheck vial #4 thoroughly and add 40uL of ORTHO Bio-Clone Anti-D. Mix thoroughly and centrifuge. This will give a positive DAT and a positive Rh control on ProVue. We use it for 7 days.
-
2 hours ago, lmunger said:
We are considering implementing computer XMs with our new Cerner Blood Bank system. I am under the impression that the retype for an electronic crossmatch must include a backtype retype? We currently don't perform backtype testing on any of our patient retypes, which are performed in tube.
Yes, if you are AABB accredited, then you must do an ABO determination (as defined in 5.14.1) on the initial blood sample and an ABO determination (as defined in 5.14.1) on the 'retype specimen'. FDA, CAP and other accrediting agencies do not prescribe how the 'retype' must be done.
-
On 1/12/2017 at 4:36 AM, Jennifer Castle said:
Yes, it's a tube drawn for Blood Bank that has no testing associated with it. It's simply ready for testing if needed. We hold them for three days.
It is a formalized process at our facility, Clot to Hold must be ordered in HIS and resulted in LIS. No charge. Based on internal data, we don't centrifuge prior to storage.
-
17 hours ago, SMILLER said:
Someone remind me why, if an initial typing is O, that it is thought to be unnecessary to retype with another specimen (for facilities who normally would draw another specimen for the re-type).
Thanks, Scott
It depends. If your goal is accuracy in ABO typing then draw a second specimen. If your goal is patient safety, then it isn't necessary to type a second specimen because you routinely will select group O donor units and they will always be compatible. At my facility, 55% of patients with no previous ABO on file type as group O.
-
2 hours ago, milesd3 said:
Unless I'm mistaken AABB changed it wording on how good a sample is good for. Years ago it said 72 hours but that was changed to read 3 days so if the unit was given within those 3 days there is no problem. Might be a grey area after midnight since technically its a new day.
That is correct. We should be thinking in terms of calendar days not hours. A calendar day starts at 0000 and ends at 2359. A specimen collected at 0000 does not expire until 2359 of that calendar day.
-
1 hour ago, mollyredone said:
Is that written in any AABB publication or inspection checklist, or is it just common sense?
AABB Standards 30th Edition 5.14.3.2 states, "The sample shall be obtained from the patient within 3 days of the scheduled transfusion in the following situations. Day 0 is the day of draw." The three conditions are 1)transfused in the preceding 3 months with blood component containing allogenic red cells, 2)pregnant within the preceding 3 months or 3) history of transfusion or pregnancy is uncertain or unavailable.
I interpret this to mean that the blood component must be issued before the sample expires at 2359 on day 3.
AABB Standard 5.28 Administration of Blood and Blood Components states, "There shall be a protocol for the administration of blood and blood components... The protocol shall be consistent with the Circular of Information for the Use of Human Blood and Blood Components. Standard 7.5 applies."
I don't have a copy at hand but it may address blood being infused after blood sample has expired?
-
46 minutes ago, mollyredone said:
I thought I had read on the forum somewhere that patients that type O with the first type don't need a second type for electronic crossmatch. Is this true? I don't know if I can override that in Meditech. Or do you need to do an IS XM? I know that if you don't have a 2nd type on other blood types, you can do an IS XM and give O blood.
Electronic crossmatch requires 2 ABO types on file regardless of the initial ABO typing. Patients who don't qualify for the electronic crossmatch may be serologically crossmatched with ABO identical or ABO compatible RBC's regardless of the initial ABO typing.
Meditech will prompt you that patient does not qualify for electronic crossmatch if no second ABO on file. I am not aware that you can override this requirement in Meditech regardless the patient's initial ABO typing.
-
I have been running the CAP Survey DAT for years on ProVue without any failures. CAP provides 3 samples in sealed vials. We thoroughly resuspend the contents of the vials and then pour the entire contents (2-3ml) of the vials into individually labeled tubes. Centrifuge the tubes for 15 seconds (in the same centrifuge we use for tube testing). Specimens are now ProVue-ready for testing.
-
To minimize the number of steps required to issue blood, we retain donor tubing segments as part of the ABO/Rh confirmation process on receipt of donor units from the donor center. I dislike trying to find the end of the donor tubing that is tightly rubber-banded together under any circumstance and certainly not when a nurse is standing beside me!
-
7 hours ago, Malcolm Needs said:
Hi Dansket,
Yes, I have seen several examples of the A3 phenotype, several examples of D mosaic and quite a few Lutheran antibodies of various specificities, all in gel, but not an anti-Sda. It may seem a bit "strange" that I can claim to have seen "several examples" of the A3 phenotype in gel, but it has to be remembered that we have been using the gel technique for well over 16 years, and that I worked (that past tense still seems really weird!) in one of the two Red Cell Immunohaematology Reference Laboratories in London, which has a huge ethnic mix, and so we saw all sorts of rare antigens and antibodies.
Please describe the appearance of the anti-A gel column when testing an A3 phenotype? Was the distribution of red cells in the gel column classic dual-population (rbc trapped on top, rbc button at the bottom, but no rbc within the gel column) or something else?
-
Edited by Dansket
added content12 hours ago, Malcolm Needs said:Okay Dansket, I accept what you are saying in terms of definition, but I still say that mixed-field reactions can be seen in gel in cases of the A3 phenotype, D mosaics (see above), many antibodies directed against antigens within the Lutheran Blood Group System, where the stringy appearance of the agglutinates seen in tube agglutination is also seen in gel, and those with anti-Sda specificity.
I'm not disagreeing that other types of serological phenomena are seen as mixed-field reactions when viewed microscopically on a slide, but can only speculate as to how these reactions are observed in gel. I've seen A3 and anti-Sda mixed-field reactions through a microscope on a slide but not yet in gel. I'm curious, have you see an A3 in gel?
-
Malcolm, please re-read my post. Mixed-field is a term used to describe the observation of a microscopic reading on a slide of the contents of a standard tube test where there may be seen "individual clumps of red cells in sea of unagglutinated red cells".
Two populations of red cells will be present in a group A recipient of a group O red blood cell transfusion. When testing a post-transfusion blood sample, a band of agglutinated red cells will be trapped at the top of the gel column, a button of red cells will be seen at the bottom of the gel column and no red cells trapped within the anti-A gel column. These observations are visible without the aid of a magnifying device and are graded as dp dual-population according to MTS-ID Gel Card Interpretation Guide used for both manual and automated gel testing.
-
-
There is a dictionary titled MIS Customer Defined Screens. Attributes are written in Magic Programming code and that is what you see above. Let me know if the attached file helps. QUERY BLOOD ISSUE.docx
-
3 hours ago, ESIZENSKY said:
Can it be barcoded? We are implementing hemonetics blood track and the Ecode is scanned to confirm the correct product.
My strategy to confirm the correct product is to add queries to the blood issue by patient routine.
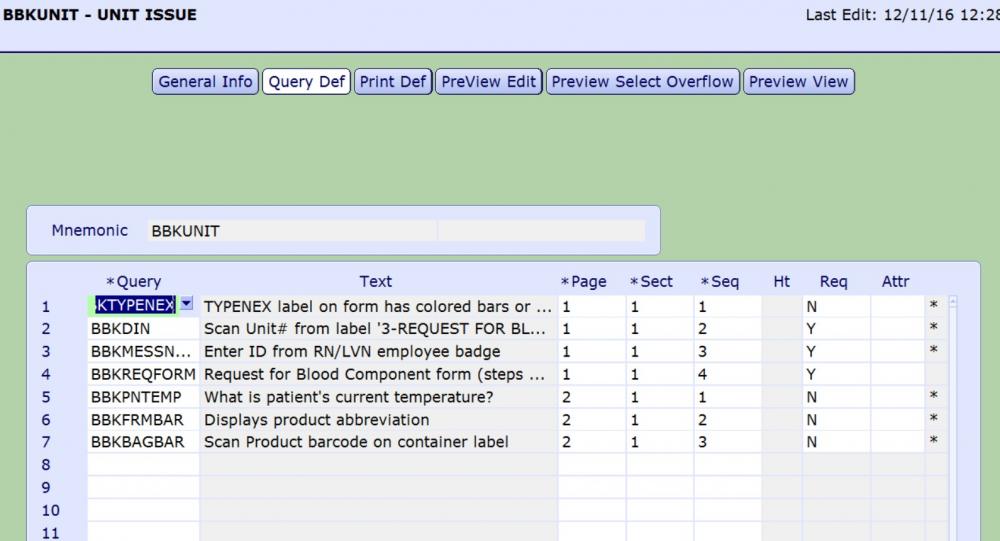
This attribute reads the product abbreviation of the component selected for issue and defaults the result into the BBKFRMBAR query.
This query requires user to scan Ecode on the blood component container label, verifies that ISBT128 barcode was scanned and then compares Ecode scanned into BBKBAGBAR to Ecode from BBKFRMBAR.
-
-
40 minutes ago, Darren said:
It's frustrating what meditech has forgotten to include in calculations and interfaces and rules. With the right keywords available it would be highly customizable and much simpler to get the system to do what you want. In the case of the way your labels printing with antibodies it would be simpler if there were a keyword in the label format dictionary that would display all antibodies. I guess that's what happens when programmers have no experience working in a lab with their system.
Do you find that you get false markers applied to patients? As in someone typos a positive ab screen, the marker attaches, then they correct it and the marker is still attached?
Darren,
Put it a service request for custom keyword. I have had some success with this approach in the past.
I have intentionally not trained staff to add or remove markers.
If a positive antibody screen entry is saved and verified, a whole lot of tests are automatically reflexed. So it is much more than just correcting a marker. I review all antibody identification workups for appropriate computer entry. It hasn't been an issue ....Yet.
-
Correct, the antibody markers are stored in MRI History. There are many other markers in BBK History and I only wanted antibody markers to print on the Meditech specimen collection labels because of limited space on the label.
We are using Meditech C/S 5.67. We don't enter antibody identification panel results into Meditech.
Per Iatric, there is no way to cause antibody markers to be added when the test ABID is resulted using the BBK Antibody dictionary.



Validating a new Freezer
in Accrediting Agencies
·
Edited by Dansket
modified sentence
Do you really read freezer temps to a tenth of a degree? A typical freezer temperature recorder chart cannot be read to a tenth of a degree nor can a refrigerator temperature recorder chart be read to a tenth of a degree.