Sherif Abd El Monem
Members
-
Joined
-
Last visited
-
Country
Egypt
-
Guide to the preparation, use and quality assurance of blood components 22
🩸 New Resource Announcement: EDQM Blood Guide – 22nd Edition Now Available (Free Download) The European Directorate for the Quality of Medicines & HealthCare (EDQM) has just released the 22nd Edition of the Guide to the Preparation, Use and Quality Assurance of Blood Components — a foundational document for professionals in transfusion medicine and blood banking. 🧾 Key Updates in the 22nd Edition: 🧴 New chapters on topical use of blood components 🆘 Expanded guidance on emergency planning and preparedness 📉 Comprehensive revisions to the haemovigilance section ✅ Updated references to current EU donation and transfusion standards This guide is a valuable reference for: Blood bank and transfusion service staff Clinical pathologists and transfusion medicine specialists Hospital policymakers and regulatory authorities Medical educators and trainees in the field 📘 We've summarized the key highlights and provided the official download link here: 👉 Guide to the preparation, use and quality assurance of blood components 22 💡 Feel free to share with colleagues, trainees, and anyone involved in blood safety and quality systems. #EDQM #BloodTransfusion #TransfusionMedicine #Haemovigilance #BloodBanking #ClinicalStandards #BloodComponentQuality
-
📚 Global Immunohematology Giveaway – 24 Hours Only!
📚 Global Immunohematology Giveaway – 24 Hours Only! 🌍 Read It in Your Language – Get the eBook Free for One Day! 🎁 Celebrate Science Without Borders! 🩸 Big News! To celebrate the global journey of my book "Immunohematology Made Easy (1): The 5-Days Journey", I’m offering free access for 24 hours to all translated versions – now available in: 🌐 Arabic | English | French | Portuguese | Hindi | Simplified Chinese 📅 This offer is valid for 24 hours only 📥 Available formats: PDF & EPUB 🛍️ Download from: https://store.immunohematologymadeeasy.net 🔁 Share this with colleagues, students, educators, and anyone interested in transfusion science! Together, let’s make transfusion knowledge accessible for everyone, everywhere. Thank you for supporting the mission! 💉📘🌎
-
Exploring Innovations in Transfusion Medicine Trove+3
Clinical Use of Blood: A Different Approach presents a comprehensive analysis of modern transfusion practices, emphasizing AI integration and clinician education. A must-read for professionals in the field. You can download it from the link below. Clinical Use of Blood: A Different Approach
-
Paper Summary & Visual Explanation of: “Adverse outcomes after red blood cell transfusion in very low birth weight infants in a resource-restricted hospital”
📄 Summary & Visual Guide: Adverse Outcomes Following RBC Transfusions in VLBW Infants – A Study in a Resource-Limited Setting This post presents a structured summary and a visual explanation of a critical study evaluating complications observed after red blood cell transfusions in very low birth weight infants at a resource-restricted hospital. 🔍 Key Insights: Common post-transfusion adverse events Operational and clinical challenges in low-resource NICUs Implications for transfusion protocols and neonatal outcomes You will find in the post: The paper explained from a teacher’s perspective Key results presented in slides for clarity Visual representations of the study’s findings A comprehensive full summary 🔗 Access the full post: Paper Summary & Visual Explanation of: “Adverse outcomes after red blood cell transfusion in very low birth weight infants in a resource-restricted hospital” 🧠 How do we bridge the gap between need and capacity in neonatal transfusion care? Join the discussion.
-
-
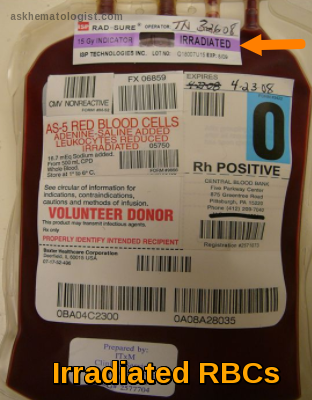
What’s this irradiation label on this bag?!!!
(This conversation is happening in the blood bank, next to the blood component fridge. 'T' is closely inspecting an RBCs bag.) Characters: 'S': Someone who has been working in the blood bank for a while. 'T': Someone who just started working in the blood bank. T: Hey, 'S', I keep seeing these bags with the "IRRADIATED" sticker on them. I understand that something was done to the bag, but why do only certain bags need this? And what exactly does it mean for the blood? S: Excellent question, 'T'! This is a very important safety step that we perform for specific patients. Let’s break it down step by step. Blood irradiation simply means exposing cellular blood components—such as RBCs, Platelets, and Granulocytes—to a controlled and calculated dose of radiation, usually using gamma rays or X-rays. T: Radiation? That sounds serious! Why do we do this? S: Exactly! Don’t just do things blindly—try to understand the reason behind them! This irradiation is an additional step we apply to blood components for a very important reason. What is it? It’s to prevent something called: Transfusion-Associated Graft-versus-Host Disease (TA-GVHD). T: Graft-versus-Host… like what happens in transplants? Can that happen from just a regular blood transfusion? S: Yes, exactly the same concept! Though thankfully, it’s quite rare. Blood components contain immune cells from the donor, specifically T lymphocytes. In TA-GVHD, these donor T cells recognize the patient’s body as foreign and start attacking it. Normally, the patient’s immune system can handle these donor cells, but problems arise when: The patient’s immune system is very weak and unable to fight back (due to certain diseases or treatments). The patient is on immunosuppressive drugs. The donor is a relative of the patient or the bag is HLA-matched. In this case, the patient’s body may not recognize the donor’s cells as foreign, allowing them to survive and proliferate, but at the same time, these donor cells attack the patient’s body. T: Got it, that makes sense. So, irradiation stops these T cells from attacking? S: Exactly! Radiation damages the DNA of the T cells, preventing them from multiplying or launching an attack. This effectively neutralizes the risk of TA-GVHD. T: So, who exactly needs irradiated blood? S: Patients with weakened immune systems, as we just discussed. The key groups include: Patients receiving intrauterine transfusions (before birth). Premature infants or low-birthweight newborns. Patients with congenital immunodeficiencies. Those undergoing stem cell or bone marrow transplants. Patients with certain cancers or those on strong immunosuppressive drugs (e.g., Fludarabine). Patients receiving granulocyte transfusions. Anyone receiving blood from a relative or an HLA-matched donor. T: Understood. So how is irradiation done in practice? S: We place the blood bag in a specialized device called a blood irradiator, which delivers a precise dose: U.S. standards: At least 25 Gy at the center of the bag and 15 Gy at any other part. European standards: Minimum of 25 Gy anywhere in the bag, but the allowed range can go up to 50 Gy. This dose destroys the T cells. We also use indicator labels that change color to confirm the bag has been irradiated, and the machines are regularly checked to ensure they deliver the exact required dose. T: Does irradiation affect the blood itself? Like its quality or how long we can store it (shelf life)? S: Good point! Yes, it does have some effects, especially on Red Blood Cells (RBCs). Irradiation causes slight damage to the RBC membrane, leading to increased potassium leakage and some cell breakdown during storage. Because of this, the shelf life of the blood bag may change. This is why we follow strict guidelines, typically based on: American (AABB) guidelines. European guidelines. But most importantly, the policy of your specific institution. Shelf Life Rules for RBCs: American Standards: Irradiation can be done at any time before the bag’s original expiration date. The new expiration date becomes: 28 days from the irradiation date OR the original expiration date, whichever comes first. Example: An RBC bag has an original shelf life of 42 days. If irradiated on Day 10, the new expiration date will be Day 38 (10 + 28) since that’s earlier than Day 42. If irradiated on Day 20, adding 28 days would bring it to Day 48, which exceeds the original Day 42 expiration, so the bag will still expire on Day 42. European Standards: Irradiation must be done within the first 28 days from donation (older bags cannot be irradiated). The new expiration date becomes: 14 days from irradiation OR 28 days from donation, whichever comes first. Example: If a bag is irradiated on Day 10, it will expire on Day 24 (10 + 14) since that’s earlier than Day 28 from donation. If irradiated on Day 20, adding 14 days brings it to Day 34, but since that’s past Day 28, the bag still expires on Day 28. T: Wow, that’s really precise! So the expiration date must be carefully checked after irradiation. S: Absolutely! Ideally, we try to irradiate RBCs as close to transfusion as possible. If an irradiated RBC bag isn’t transfused within ~24 hours, it’s often recommended to wash the bag before giving it to the patient. This is mainly to remove excess potassium that may have leaked. T: What about platelets? Does their shelf life decrease too? S: Luckily, no. Platelets tolerate irradiation much better. They can be irradiated at any point within their shelf life and still keep their original expiration date. T: One last question? S: Ask away, my friend! T: Doesn’t leukoreduction also prevent GVHD? S: That’s a common question! Leukoreduction significantly reduces the number of white blood cells, but it doesn’t eliminate them completely. Some T cells may still remain in amounts that can cause TA-GVHD in high-risk patients. That’s why leukoreduction is NOT considered a replacement for irradiation when the goal is to prevent TA-GVHD. However, some newer pathogen inactivation technologies treat blood components to reduce infection risks, and these methods also disable T lymphocytes. So, blood components that undergo pathogen reduction often don’t require irradiation to prevent TA-GVHD. T: This is super useful, 'S'! Now that I understand why we irradiate blood and how it affects storage, everything is much clearer. It’s clearly a critical step for patient safety. S: Exactly! It’s all about protecting patients with weakened immune systems. And always keep asking questions, 'T'—that’s the best way to learn in the blood bank! What’s this irradiation label on this bag?!!!
-
Mastering the DAT: A Deep Dive into 'Current Applications and Interpretations of the Direct Antiglobulin Test' (AABB, 1988)
📖 Book Review & Discussion: Revisiting the Direct Antiglobulin Test (DAT) – AABB 1988 The Direct Antiglobulin Test (DAT) remains crucial in immune-mediated hemolysis diagnosis, but its interpretation has evolved. This review examines the AABB publication Current Applications and Interpretations of the DAT (1988) and its relevance today. 🔬 Key topics include: 🩸 The predictive value of a positive DAT in different populations 🩸 Differentiating autoimmune vs. alloimmune hemolysis 🩸 Classic methodologies vs. modern techniques (solid-phase, flow cytometry) 🔗 Read the full review: [Mastering the DAT: A Deep Dive into 'Current Applications and Interpretations of the Direct Antiglobulin Test' (AABB, 1988)] How do you approach weakly positive DATs? Have testing advancements changed your diagnostic approach? Let’s discuss!
-
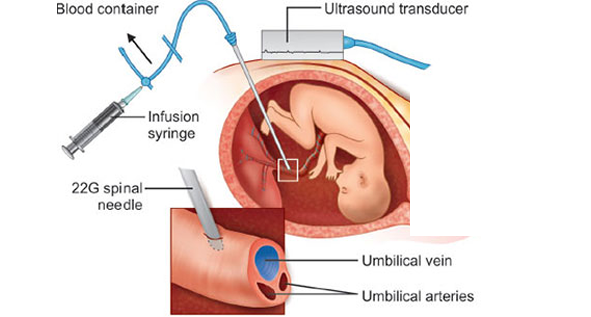
Come on, we have an IUT case!!!
Come on, we have an IUT case!!! In one of the blood banks, this conversation took place between: ‘S’ – someone who has been working in the blood bank for a while. ‘T’ – someone who just started working in the blood bank. S: Ready, dear? Come on, we have an IUT case! T: IUT? What does that mean? I’ve never heard of that before. S: Alright, let’s break it down step by step. IUT stands for Intrauterine Transfusion, which is the process of transfusing blood to the fetus. T: Fetus? You mean the baby after birth, right? S: No, just as you heard—it’s a blood transfusion to the fetus itself while still inside the womb. T: That’s very strange! Why would a fetus even need a blood transfusion? S: The most common reason is severe fetal anemia, which usually happens due to HDFN. Do you remember what HDFN stands for? We talked about it before. T: Yes! It stands for Hemolytic Disease of the Fetus and Newborn. S: Exactly! Well done! You just said hemolytic disease of whom? The fetus! So it’s not just about newborns, right? T: Right, right! Got it. S: Maternal antibodies cross the placenta and attack antigens on the fetal red blood cells, binding to them and leading to hemolysis. T: Okay, but something is confusing me—how do they even know the fetus has anemia? They can’t just draw blood from its arm and run a CBC, right?!!! S: Hahaha, of course not! Fetal anemia is mainly diagnosed using Doppler ultrasound, which measures blood flow in a fetal artery called the middle cerebral artery (MCA). Since anemic blood is “lighter,” it moves faster. By comparing the blood flow velocity to gestational age, they can determine whether the anemia is mild or severe. If there is a high suspicion, they can take an additional confirmatory step by performing cordocentesis, which involves drawing a direct blood sample from the fetal umbilical cord. T: So, if the Doppler confirms anemia, what happens next? S: At that point, IUT is usually necessary. The goal is to treat the anemia and prevent serious complications for the fetus. T: Got it! So, how is IUT performed? S: The procedure is done under sterile conditions, guided by ultrasound. A needle is inserted through the mother’s abdomen into the umbilical vein, and compatible red blood cells are transfused directly into the fetal circulation. This is the most common method of performing the procedure. T: How much blood is transfused? S: The volume depends on multiple factors, such as fetal weight and anemia severity. There’s a well-known formula: Blood Volume Needed=Fetal Weight (g)×0.14×(Desired Hct−Pre-transfusion Hct)/RBC Unit Hct Let’s break it down: Fetal Weight (g): Obtained from ultrasound. 0.14 mL/g: An estimated value representing fetal blood volume per gram of fetal weight. Desired Hematocrit (Hct): The target post-transfusion hematocrit, usually 40-50%. Fetal Hematocrit (Hct): The current hematocrit level before transfusion. Hematocrit of Donor Blood: Usually 70-85%. For example, if: The fetus weighs 1000 g The desired hematocrit is 40% The pre-transfusion hematocrit is 15% The donor unit hematocrit is 85% Then: Blood Volume Needed=1000×0.14×(0.4−0.15)/0.85=41.2 mL S: Great! Now, what about the blood itself? What are the requirements? T: The requirements we follow might differ slightly from those of some of our colleagues in other places, as they adhere to their institution’s standards. For the mother’s sample: Determine blood type. Perform antibody screening. If positive, do antibody identification to determine which antibody is present (since it’s likely causing HDFN). The transfused unit must be negative for the antigen targeted by the maternal antibody. If the mother has a previously recorded antibody, we check if any new ones have developed. For the red blood cell (RBC) unit: Blood type: O-negative. Must be compatible with the mother and antigen-negative for any maternal antibodies. Washed . Leukoreduced . Irradiated . CMV-negative. Hemoglobin S-negative. Fresh unit (preferably 5-7 days old). Hematocrit: 70-85%. Anticoagulant preservative solutions: Preferably CPDA-1 (or SAGM if washing is available). S: So, we need a meticulously prepared RBC unit—it’s not just any random unit! T: Exactly! This is extremely important, and these blood unit specifications are critical for the procedure. We need to work quickly, efficiently, and with absolute precision. S: Thank you so much! I’ve really learned a lot today. T: No problem, dear! May we always be a source of help and healing for others! Come on, we have an IUT case!!!
-
Mixed-field ABO front typing as an early sign of disease recurrence in ABO-matched stem cell transplantation-American Red Cross webinar
🔬 Mixed-Field ABO Front Typing as an Early Sign of Disease Recurrence in ABO-Matched Stem Cell Transplantation A recent American Red Cross webinar highlights the significance of mixed-field ABO front typing in detecting early disease recurrence post-transplant. 🩸 Key Insights: 🔹 Mixed-field reactions could indicate relapse or donor-host interactions 🔹 ABO typing may serve as a valuable monitoring tool in transplant recipients 🔹 Clinical implications for transfusion management & post-transplant care This engaging session will feature the article’s authors, esteemed experts Nalan Yurtsever, MD and Christopher Tormey, MD from Yale School of Medicine. They will lead a live discussion of their research and findings. The session will be expertly moderated by Janis R. Hamilton, MS, MLS(ASCP)SBB How should transfusion medicine specialists interpret mixed-field typing results in transplant patients? Let’s discuss the clinical significance! Mixed-field ABO front typing as an early sign of disease recurrence in ABO-matched stem cell transplantation
-
 Malcolm Needs reacted to a post in a topic:
Options for Pre transfusion Testing – Where Does Tube Testing Fit(Webinar)- Susan T. Johnson
Malcolm Needs reacted to a post in a topic:
Options for Pre transfusion Testing – Where Does Tube Testing Fit(Webinar)- Susan T. Johnson
-
Options for Pre transfusion Testing – Where Does Tube Testing Fit(Webinar)- Susan T. Johnson
🔬 Pre-Transfusion Testing: Is Tube Testing Still Relevant? 🩸 In a world of automation, is there still a place for test tube methods in transfusion medicine? The answer is YES! Here’s why: 🔹 Resolving ABO Discrepancies – When automated methods flag uncertain results, tube testing helps confirm patient blood type. 🔹 Detecting Weak D and Partial D Phenotypes – Some Rh discrepancies can only be resolved using test tubes or additional reagents. 🔹 Uncovering IgM Antibodies – Many automated platforms focus on IgG, but tube testing catches clinically relevant IgM antibodies. 🔹 Handling Urgent Transfusions – When time is critical, tube testing can provide faster results than automated methods. 🔹 Troubleshooting Complex Cases – From mixed-field reactions to multiple antibodies, tube testing remains a trusted problem-solving tool. ⚠️ Takeaway: While automation enhances efficiency, test tube methods are still vital for accurate pre-transfusion testing and patient safety! 🎥 Watch the full webinar by Sue Johnson. 📄 Get access to: ✅ Webinar Transcript ✅ Bullet-Point Summary of Key Takeaways ✅ Q&A Session from the Webinar ✅ Study Based on the Webinar Transcript 💬 What’s your lab’s approach? Do you still use tube testing? Share your thoughts! 👇 Options for Pre transfusion Testing – Where Does Tube Testing Fit(Webinar)- Susan T. Johnson
-
BloodBankTalk: Pathogen inactivation or reduction
I just answered this question. My Score FAIL
-
HemeLabTalk: Multiple Myeloma
I just answered this question. My Score PASS
-
-
ISBT Corporate Partner Webinar: Arboviruses and transfusion safety
🔬🩸 Are you concerned about arboviruses impacting blood transfusion safety? Join ISBT & Grifols Diagnostic for a FREE webinar on February 25th, 4-5pm CET! 🗓️ Expert speakers José Eduardo Levi & Xiaomei Zhu will discuss the emerging threats to the global blood supply. Perfect for scientists, blood bankers, public health pros, and students! Don't miss out on this crucial discussion! ISBT Corporate Partner Webinar: Arboviruses and transfusion safety
-
-
Immunohematology Made Easy group
🌟 Join Our Immunohematology Community! 🌟 Peace and blessings be upon you all. I’m excited to invite you to join my Telegram group Immunohematology Made Easy – a space dedicated to learning and sharing knowledge about blood transfusion medicine. Whether you're a beginner, an experienced professional, or an esteemed expert, there's something for everyone in our group! In this community, we focus on: Learning Together: From the basics to advanced concepts in blood transfusion, we’re here to learn and share. Open Discussions: Ask questions, share insights, and help simplify complex topics. Supportive Environment: Everyone’s contribution matters, regardless of your level of experience. This group is a platform where: Newcomers can learn at their own pace. Experienced professionals can share their expertise. Everyone can join in discussions and grow their understanding of this critical field. Let’s connect, grow, and contribute to advancing transfusion science together! If you're passionate about this field, feel free to join and invite others who might benefit. 📲 Join us: Immunohematology Made Easy group
-
-
 Malcolm Needs reacted to a post in a topic:
Basic immunohematology : testing in tube by Dr.Sue Johnson
Malcolm Needs reacted to a post in a topic:
Basic immunohematology : testing in tube by Dr.Sue Johnson
-
Basic immunohematology : testing in tube by Dr.Sue Johnson
🧪 Dive into the world of immunohematology with Sue Johnson's must-watch video series! 🧪 Learn essential tube testing techniques, including ABO and Rh typing, antibody detection, and more. These videos are perfect for anyone looking to master the art of serology. Filmed at the Blood Center of Wisconsin and produced by Dr. Jim Perkins of the Indian Immunohematology Initiative. Don't miss out on this valuable resource! 🌟 Basic immunohematology : testing in tube
-
ISBT/Bio-Rad Webinars-Could it be drugs? How to differentiate AIHA from DIIHA
📢 Join this for an insightful webinar on understanding autoimmune hemolytic anemia (AIHA)! 🩸 Learn how to differentiate between warm and cold AIHA, and how these conditions can mask drug-induced immune hemolytic anemia (DIIHA). 🔍 With expert speakers, Susan T. Johnson and Arnaud Reggiani, will guide you through the serologic characteristics and testing methods. Don't miss out! ISBT/Bio-Rad Webinars-Could it be drugs? How to differentiate AIHA from DIIHA


.thumb.jpg.1c2217b1e436efe5f371976f7cec58d4.jpg)