Posts posted by AB123
-
-
Just want to see how many labs out there use flying squad blood and if so how do you manage it? does your LIS have a process built in for managing such situations or do you have a manual process where the documentation is resolved later?
Thanks
-
Maybe I'm reading your question wrong but why do you need to use XM profile? we do antigen typing for all antigens on our vision and the Vision has profiles setup for all the sera it offers. Ortho also has the option for other manufacturers sera and these can be setup in the UDP (User defined protocols). You have to titre the sera to ensure it has a tire of less that 1:1024 as this is what Ortho claim is the limit of their analyser for carry over.
-
-
10 hours ago, David Saikin said:
Did you test your A2 cells in gel or in tube? If in tube, did you look at it microscopically?
The A2 was done in Gel, or rather glass beads as Ortho is out here. The left well is A2 and the right is A1 so clearly weaker reaction with A2 but still reacted. I also repeated the tube method and when I did it, A1 gave strong reaction and the A2 was barely visible by eye by but large agglutinates observed microscopically.
Any suggestions for further tests I can do on this one? or would genetic testing be the only way to resolve this now?
-
-
2 hours ago, Malcolm Needs said:
Did you really mean "an Anti-A", and not an Anti-A1? Surely, if the A antigen is expressed on the red cells, however weakly, the patient cannot produce an anti-A, unless it is an auto-antibody?
In your experience does the fact the Anti-A is reacting with A2 cells rule out A subgroup of A been present?
-
2 hours ago, Malcolm Needs said:
Did you really mean "an Anti-A", and not an Anti-A1? Surely, if the A antigen is expressed on the red cells, however weakly, the patient cannot produce an anti-A, unless it is an auto-antibody?
I meant it in the sense that it's an antibody reacting with the A antigen rather than another cold reacting antibody reacting with another antigen on the Acells been the cause.
However as per my original post it did react with A2 cells this along with the strength of the back group was why I was questioning and AsubB.
-
-
AABB mandates the giving set are Pyrogen free, it appears the ones we use are Endotoxin free however they have advised that this is not in compliance with the standard as not all Pyrogens are endotoxins. Just curious what giving set other site that are AABB accredited are using?
Thanks
-
-
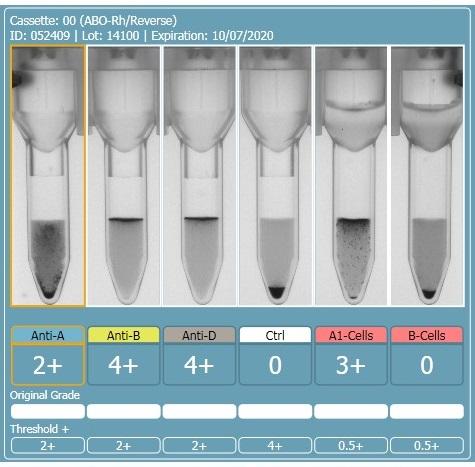
We have a case at the moment, a patient with no history, blood group by Ortho Cassette method is Anti-A 2+, Anti-B 4+, Anti-D 4+, CTRL Neg, A1 Cells 3+, B Cells Neg.
Tube group gives B Pos and the Anti-A is totally clear Microscopically not even any apparent rouleaux, however when repeated at 4°C some small agglutinates were visible. Washing the cells yielded no change in the Anti-A reaction and auto control is negative in poly cassette as is the Antibody Screen. Back group tested with A2 cells also Positive.
Is there any further tests that should be considered to try to ascertain the correct blood group? If not how would you report the blood group?
Is this likely an A Subgroup B? If so would genetics be the only way to confirm this?
Thanks
-
-
3 hours ago, AMcCord said:
Generally a new infusion set is required after 4 hours. If infusion of a second unit will pass the 4 hour mark on the initial infusion set, a new set should be used when the 2nd unit is started.
Do you have any reference for that? I was under the impression a giving set could be used for up to 3 units of PRBC and only needed to be changed if there is a delay between one unit and the next. Cant find the reference for this at the minute but I'm pretty sure this is stated in either CAP or JCI. Just check AABB and they don't seem to have any standard on it.
-
The AABB standards state5.1.6.5.1 A unique identification shall be affixed by the collecting or pooling facility to each unit of blood, blood component, and attached containers, or a tissue or lot. This identification shall not be obscured, altered, or removed by facilities that subsequently handle the unit.
If I understand this correctly this means that the original DIN of all received components must not be over written. When reconstituting PRBC's for exchange transfusion, ISBT guidance allows you to either assign your own facility DIN or retain the DIN from the original PRBC unit. If you issued your own DIN does this standard mean you must still show the original DIN? Could this not cause confusion with medical staff during checking as to why 2 DIN's are displayed? If you retained the original DIN, the product code would need to change and the expiry date, is it acceptable to just cover over these portions of the original label with the new parts of the label? (product code and description and expiry date) Leaving the original DIN and blood group visible on the label?
-
-
I've been looking for some guidance on the disconnection and re-connection of blood transfusions, the only thing I have found so far is that if there is a delay between giving two components then the giving set must be changed. Which would imply the same if a bag was to be disconnected then reconnected and I would have thought removing the giving set from the now opened port would be a contamination risk?
Is anyone aware of any other standards that this would fall under?
Regards Steve
-
We have received the following response from our AABB self assessment;
1. Std 1.3: It is not clear from documents submitted how your facility will transition to the new editions of the AABB Standards for Blood Banks and Transfusion Services. Please submit an SOP.
We were requested to submit an SOP that covers transition to new versions of standards, so I sent our document control SOP that covers regular review of accreditation standards and updating policies and procedures when updates occur. I may be taking a too simplistic view of this but I don't really get what they are wanting. Or is this more to do with change control?
Can anyone advise what they have in their SOP that covers this standard?
Thanks
-
-
Edited by srichar3
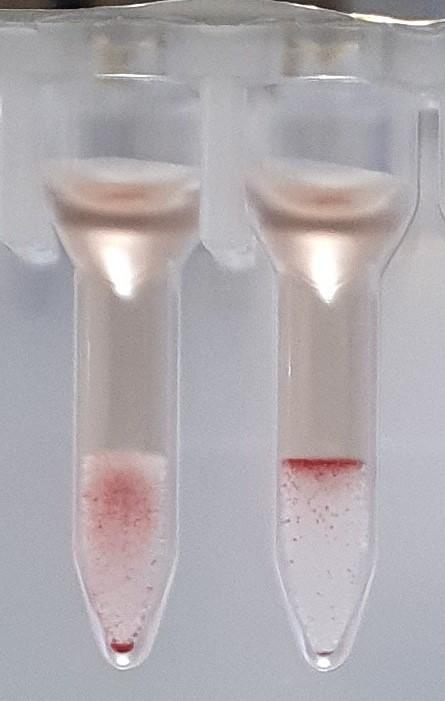
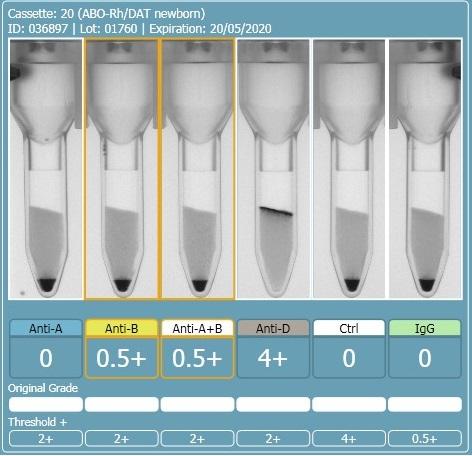
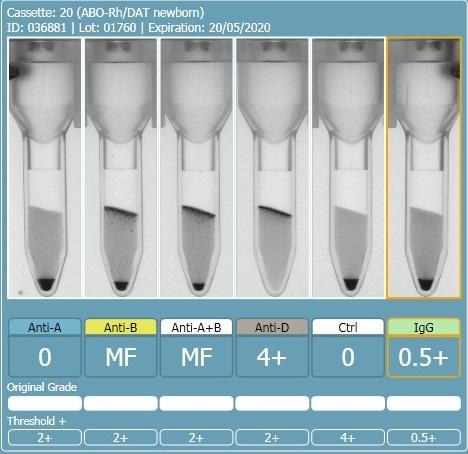
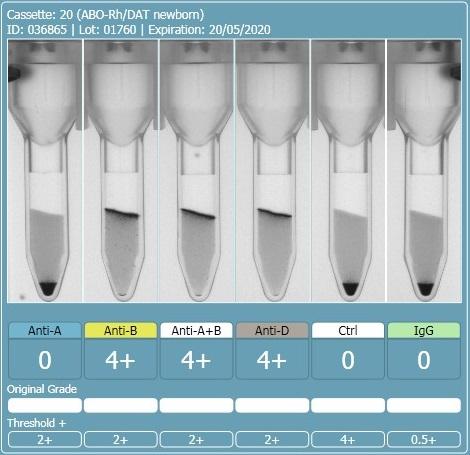
2 hours ago, R1R2 said:On the other 2 cards, this looks like a case of the B antigen not fully expressed at birth and therefore giving weak (mixed field like) reactions. The difference in strength with the last 2 could be that there was some incubation of cells and sera prior to spinning. If you really want to do more work to determine if this is contamination, you could do some Rh phenotyping (just for fun) but mom and baby would have to have different Rh phenotypes for this to work. Rh is fully expressed at birth so there should be no mixed field. I am sure there are other ways to investigate contamination and I am sure others will chime in. What is APT (last pic)?
The first and second cassette are the same specimen, the only difference was the washing of the cells between the first and second runs. I don't have an explanation as to why washing the cells changed the reactions from 0.5+ to mixed field but it seems to have increased the strength of the group B cells.
There should be no incubation of cells and sera, it was performed on an analyser and they are immediate spin, even if slight delay in centrifuging the sera and cells should be separated until centrifugation.
The APT test is alkaline denaturation, identifies cord blood from adult blood. The first specimen definitely had adult blood in it.
-
Sorry to resurrect an old thread but as my question has already been discussed in this thread I thought it worth continuing here.
I want to know if anyone has a technical explanation as to how a large amount of maternal contamination of a cord sample can occur?
In the UK in my experience at all labs I've worked its always been practice to perform APT (NAOH) test on all cords that give the same blood group as the mother, I've always seen this as one of those tests you just do but seems a bit of a waste of time as its never positive.
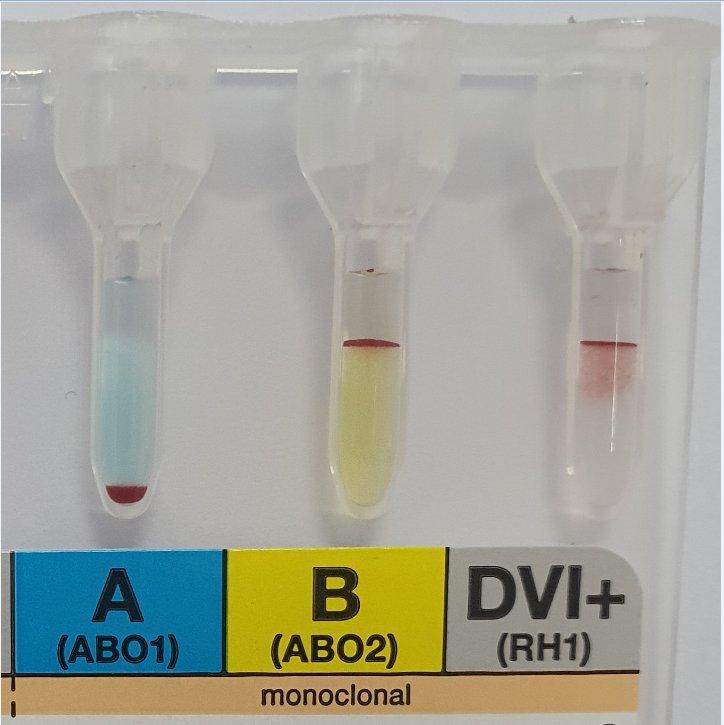
Last night we had a cord sample giving group O Pos result but with weak reactions in the B and AB wells.
I advised the staff member to wash the cells and repeat, this then showed a strong mixed field, O and B. We requested repeat venous blood which was B Positive the mothers blood group is O Pos.
APT test showed the first sample was not, or at least not all Neonatal blood.
I've done a bit of a google search and although there are a lot of papers discussing contamination there isn't much that describes exactly how it occurs. I'm assured the samples are taken by double clamping then taking the blood with a syringe and needle.
Thanks
-
-
There one and the same thing, the difference been in the UK the lab staff are not involved in the collection process like seems to be the requirement under CAP. For example we have remote fridges, when the lab staff put the units in the remote fridge ready for the nurses to collect this is issued. Does that not fit your definition above of distribute? But at this point it is still not collected by the Nurses and may stay in this fridge for upto 48 hours before return to the lab if not collected. When we "issue"blood in the UK it is made available for the nurses, they then complete the final checks and sign the blood out alone not together with lab staff.
-
I got into a bit of a debate today in a meeting with our IT regarding our blood bank module and wanted to see what others opinions are on what blood "issue" means. They were of the opinion that issue means the time the product is handed over to the nursing staff (collected), I disagreed as in my experience from working in the UK we consider issued the point at which the blood is assigned to the patient, crossmatched, labelled up etc and put in the fridge ready for collection. Collection is therefore as separate process not the same as issued.
I do notice however that in the CAP standards there is no mention of recording time of collection from the lab by the nursing staff, only the time of issue so wondered if in other parts of the world issue is considered the collection process?
-
I am just about to submit our self assessment for AABB, and wanted to know if anyone has any recent experience to share about the process for first time accreditation with AABB. When the inspection comes what should we expect? Regarding number of assessors, over how many days, what areas of the lab and the hospital will they want to inspect and see records from. Are the example questions in the self assessment a good representation of what they expect to see?
Thanks


Flying Squad Blood
in Transfusion Services
O Neg that is kept in a fridge that nurses/doctors can take in urgent situation without been crossmatched or issued to a patient.