Posts posted by Lcsmrz
-
-
-
From Wikipedia:
The emissivity of a material (usually written ε or e) is the relative ability of its surface to emit energy by radiation. It is the ratio of energy radiated by a particular material to energy radiated by a black body at the same temperature. It is a measure of a material's ability to radiate absorbed energy. A true black body would have an
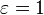 while any real object would have
while any real object would have  . Emissivity is a dimensionless quantity, so it does not have units.
. Emissivity is a dimensionless quantity, so it does not have units.Years ago, I got the 0.95 number from several industrial web sites for clear PVC plastic at 0 C, and it was used as a criteria for temp gun selection and configuration. That number was confirmed over the years in various articles about infrared thermography.
In the real world, I found that the temp guns accurately measure a unit's surface temp as a surrogate for core temp. Surface temp changes very, very quickly once the unit is removed from a refrigerator or cooler, hence the need for a more accurate confirmatory method if out-of-range.
I think there has been several posters at the AABB annual meeting over the past few years aobut these devices..
-
-
We occasionally get a sample label with the first character of the last name omitted, but all other informaton is readable. Fortunately, there is an abundance of other information on the label to affirm the patient's identity, and we call the unit manager to fix the printer immediately.
We're switching soon to a bedside scanner to confirm all specimen labels. I'd like to have the ones that print a bedside label only after scanning the patient wristband, but they're very expensive.
-
Our TAR system asks that the patient writband plus all 4 unit barcodes be scanned for the bedside verification. The "Veify" step starts the transfusion process in the computer.
For FFP and cryo, the product barcode and the exp date/time must change after thawing, hence the need for an ISBT label overlay to make sure all 4 barcodes will pass the bedside verification.
You can turn off the bedside scanning for products and exp dates, but it must be done for all units, reducing the effectiveness of the bedside verification.
-
-
We implemented TAR a few months ago at our small facility, using exisiting patient wristbands that already contained a barcoded visit number. The nurses love it, our paperwork virtually disappeared, and very few problems were encountered. We just had a CAP and JCAHO inspections within the past two months, and both inspectors were impressed with the implementation.
One encountered problem was the lack of bedside verification of RhIg injections. We code them now as Medications, rather than a BBK product.
One caveat: you must relabel components with an ISBT label for the system to work properly.
-
I think it would depend how long the thawed cryo would sit at RT.
We use a >30 min time period as significant for everything, just for consistency. Under 30 mins of room temp storage would be OK at our facility without monitoring; over 30 mins would require documentation that RT was within limits.
As with everything blood bank, you could carry this to an extreme: do you monitor the temp during transport to the floor and in the patient room before allowing a return to the Blood Bank? -- we woudn't even consider doing that, unless forced to by the FDA. What about the RT in the components, labeling or crossmatch area, when units might sit for more than a minute or two outside the refrigerator? And ad nauseum ...
-
-
I work at a 107-bed facility, and the assigned BB Tech doesn't have enough work to keep busy the entire shift. Maybe half-time on an average day shift M-F. We generrally have only one evening, night and weekend tech for the entire lab.
I think alot depends on the acuity level and the patient population. We are almost enirely computerized, whih reduces paperwork to a minimum.
-
Someone told me a story during an assessment about Biotest reagents, when one ran out before the other, even though they are used in tandem. He found that one vial's dropper had slightly larger drops, causing it to run out before its companion.
Sorry, don't remember any more details than that. At my age, I'm lucky that I remembered the event!
-
We use a Max/Min thermometer to show that our reagent storage refrigerator stays within mfr stated range.
Interestingly, it was the use of this thermometer that caused us to change refrigerators, when the old one was found to repeatedly go in- and out-of-range throughout the day through cycling and use. Once a day was giving us a false reading of the cabinet's temperature.
We also use them in refrigerators that are not monitored over weekends, to show that the temperature was maintained while we were gone.
-
Audit schedules need to be customized to each site, since the scope, equipment, personnel, past problems, etc differ substantially. How extensive the audits will need to be will also depend on the facility and its history.
We use the AABB Techniocal Manual recommendations and the manufacturer's documentation as the starting point, then progress to a point where most of us can sleep well at night. We revisit the schedule annually with the QP review.
Perhaps a better definition of "everything" would help. For example, I review employee training files annually, but QC and equipment records get audited daily.
-
-
-
It would depend what we are using the weights for ...
If weighing food sizes in the break room, we would not. If we are checking our scales used to weigh blood products, we do.
We recertify all of our measuring systems according to the schedule in our Quality Plan. Our brass weight set is recertified annually by the same outside agency that recertifies out balances.
-
-
-
-
-
-
Computer validation needs to be done on-site. If they want to come to your hospital and do it for you, there is nothing in the regulations I can think of that forbids it.
However ...
There should be a plan that is reviewed and accepted by you as comprehensive enough prior to validation. Simply accepting whatever they give you as a validation would not be acceptable for me -- it could be merely testing they require for installation and not a validation at all.
There should be documentation that they actually performed what they said they would do. That implies completed test plans, screen prints, etc. Everything should be dated and initialed.
There should be a summary of the work they did, any discrepancies that appeared in the testing and their explanation, orginial database configurations, and so on. The documentation shoulld be organized, so you and an average inspector could determine whether the plan was carried out.
The entire document must be reviewed by your facility and pronounced complete before used for patient testing.
One caveat: I learn an awful lot when doing validations. You would be depriving yourself of alot of good knowledge about the system operation and its limitations. I vote for doing it yourself!
-
-

cerner Millennium Emissivity on Infrared Therm
in Computer Systems / Software / ISBT128
As a confirmatory method, we place an NIST-calibrated LIG thermometer between two units and replace it in the cooler. We read it after 10 mins, twice the temp stabilization time of the thermometer.
Almost all temp gun failures are tech-induced -- they wait too long after removing from cooler to take the temp, and the surface temp has already started to change.