Content Type
Store
Profiles
Forums
Blogs
Events
Frequently Asked Questions
Gallery
Downloads
Glossary
Links Directory
Questions
Jobs
Vendors
Posts posted by SbbPerson
-
-
On 12/16/2022 at 11:14 AM, Bijoux71 said:
Good afternoon, I hoped to weaken or destroy unexpected (w+) reactivity observed with [2 of 6] cells at LISS-IAT in allo-adsorbed plasma. The CDP (Chloroquin Diphosphate) treated (120 min) cells appeared to have enhanced the plasma reactivity to (2+) at LISS-IAT. Obviously, there is known info about antigens sensitive to CDP treatment but I have not found any mention of blood group antigens enhanced with this chemical. I may try to repeat the CDP treatment with a shorter incubation time, or a 1:3 (cell: CDP) ratio in case it is an overtreatment issue.......Any thoughts or suggestions?
Hello, can I ask if the patient's DAT was positive for IgG?
-
-
Compared to the previous model , the ULtra CW , it is indicated that the variation of fill is just a bit higher. We use this model. In our QC/maintenance book, it indicates:
For the Ultra CW (12 tubes) , the expected fill volume is 37-40 mL. For the Ultra CWII, expected fill volume is 34-38 mL. Notice there is a higher fill variation for the CWII.
-
I was an MLT student at Walter Reed Army Hospital. At that time, there were lab techs there that were enrolled in the George Washington University SBB program. They did their blood banking clinicals at Walter Reed. I heard the whole class passed the SBB(ASCP) exam after the course was over. There were only 4 or 5 of them, and they were all Officers in the Army. Every time I see them, they would be studying. The military put extra pressure on them passing the SBB exam because they are paying for their school, salary, and room & board.
-
It's a lot cheaper than most blood bank books you see these days. Most of its reviews I have seen are positive. It appears that the first and only edition of this book was published in 2014. I think it is great that it also includes an instructor's manual, lesson slides, and test bank questions.
-
Per AABB, ABO and Rh type MUST be determined before any blood product transfusion (RBC, whole blood, Platelets, etc.), with exception only for emergent situations. Pretransfusion tests for allogeneic and autologous transfusions shall include ABO group and Rh type on the patient sample.
Sources:
Cohn, Claudia S., Delaney, Meghan, Johnson, Susan T. and Katz, Louis M.. <em>Technical Manual, 20th edition</em>. https://ebooks.aabb.org/pdfreader/technical-manual-20th-edition50155278
Standards for Blood Banks and Transfusion Services, 33rd Edition, effective April 1, 2022 (Published: 9/9/2022
-
I know this post is over 1 year old, but I have AABB books for on PBM and here is the
general strategy for implementing a PBM plan:
1) Develop and preoperative hemoglobin optimization program.
2) Develop an intraoperative red cell recovery program, and/or programs in hemodilution, component sequestration, and platelet gels.
3) Implement point of care (POC) monitoring within the operating room environment.
4) Establish an auditing mechanism to ensure reasonable blood utilization.
5) Establish a program to educate health care providers on transfusion issues.
Here are the general methods used to implement and sustain a PBM:

Sources:
AuBuchon James P., Puca, Kathleen E., Saxena, Sunita, Shulman, Ira A. and Waters, Jonathan H.. (2011). <em>Getting Started in Patient Blood Management</em>. https://aabb.ipublishcentral.com/pdfreader/getting-started-in-patient-blood-management
Cohn, Claudia S., Delaney, Meghan, Johnson, Susan T. and Katz, Louis M.. <em>Technical Manual, 20th edition</em>. https://ebooks.aabb.org/pdfreader/technical-manual-20th-edition50155278
-
-
14 hours ago, monopolova said:
Hello all,
Any differences between Ortho Panel A versus Ortho Panel B which would argue that a CLS should favor (from a serology perspective) using Panel A first to work up an antibody ID (as opposed to using Panel B first)? I imagine that if you statistically rule out your antibodies using either Panel, you should be good. But, perhaps there is a nuance I am missing.
Thanks!
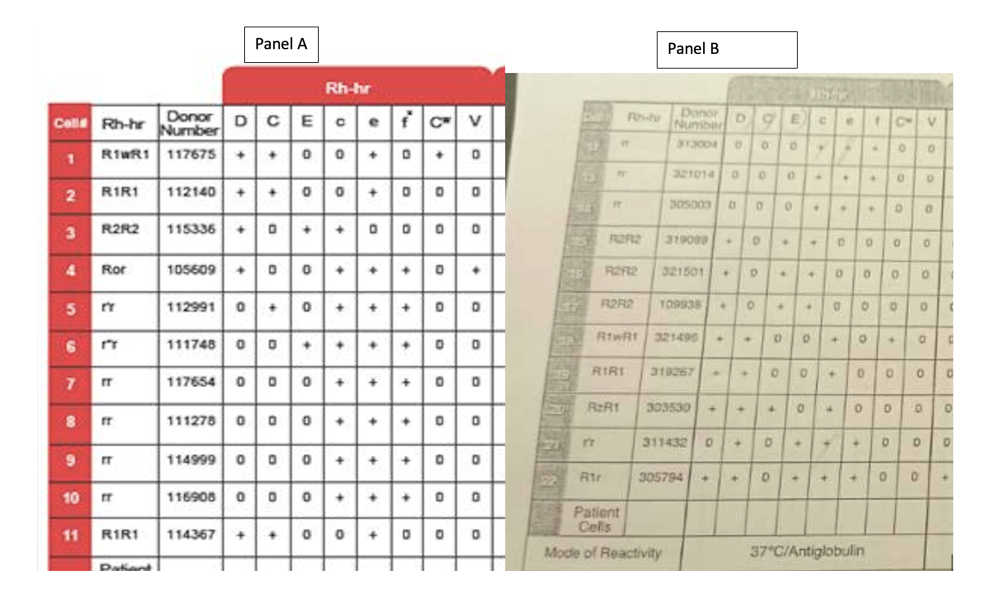
Panel B is meant to be used as a supplement to Panel A, not instead of Panel A (see inserts). Panel A is your primary panel. You only use Panel B if you have more than 1 antibody, need rule outs/ins, etc.. Part B is like a logical continuation of Part A. That is why cells are numbered 1 to 22. Panel A has cells 1 to 11. Panel B has cells 12 to 22.
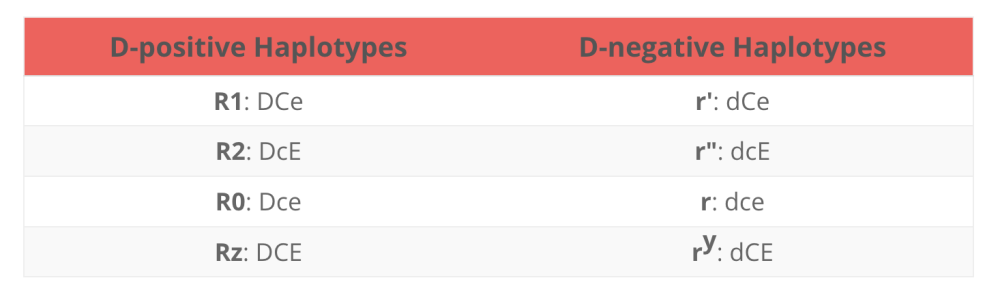
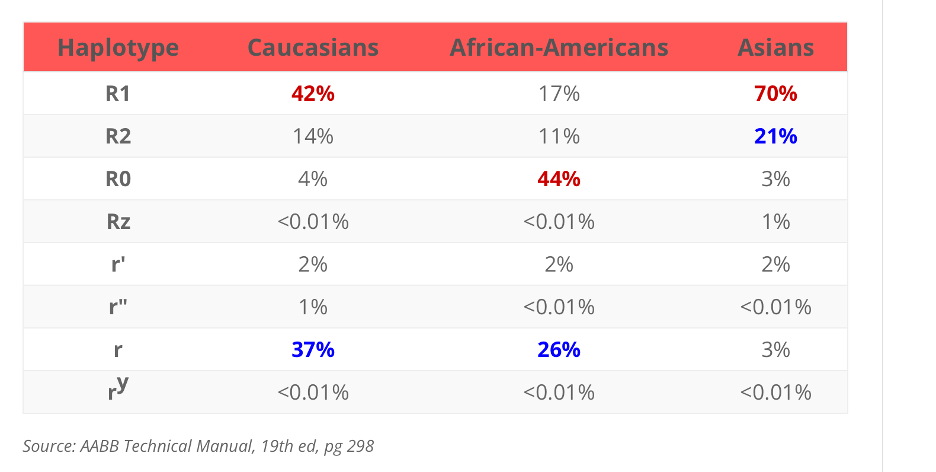
If you take in account the Rh haplotypes:
and the most common Rh haplotypes:

You can logically deduce that, the most common haplotype for caucasians are Rh positive haplotype "R1" and "R2" combos and ends with not as common haplotypes combos.
Then if you do a side by side comparison of Panel A and B, you can see that it starts out with the more common haplotypes(R1) combos and ends with the not as common combos.
You will probably won't see too much Z's, Y's and double primes (") haplotypes used in these Panels because those donors are not as common and procuring them may not be commercially feasible.
I am sure somebody smarter than me will probably prove me wrong, but these are my evidence-based thoughts on this matter.
Sources:
https://www.bbguy.org/2016/05/13/rh-blood-group-terminology/
Package Insert - Resolve Panel A.pdf Package Insert - Resolve Panel B.pdf
-
On 11/16/2022 at 3:37 PM, Cliff said:
Thank you for the reference. Do you own the book?
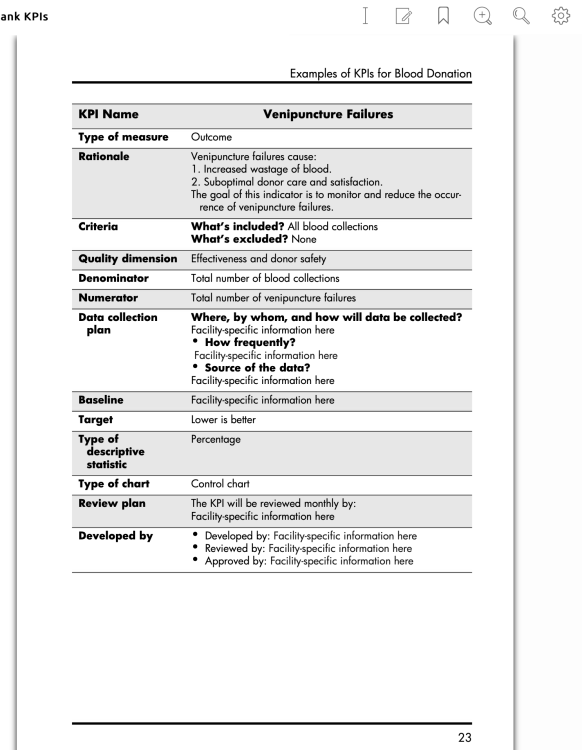
We have lots of great, actionable KPI's for donor collection, production, recruitment.
I am looking for some for regulatory services. I've done this for a long time, I struggle to come up with meaningful KPIs that we can monitor AND act on.
Yes, I own the book. It's a small book, mostly has examples of KPI's. Here is the table of contents:
Do you see anything you may find useful?
-
AABB has a Blood Donation Site Locator! Find the closest Blood Donation center to you any where in America!
https://www.aabb.org/for-donors-patients/give-blood
Please help save a life. Thank you
-
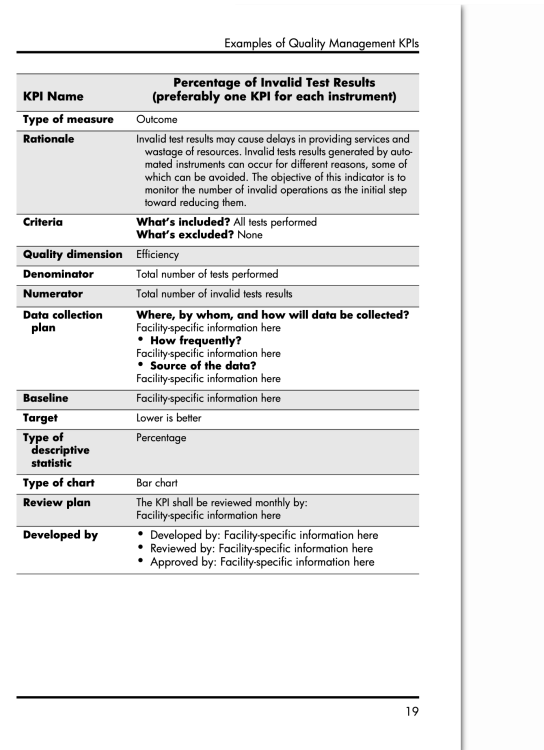
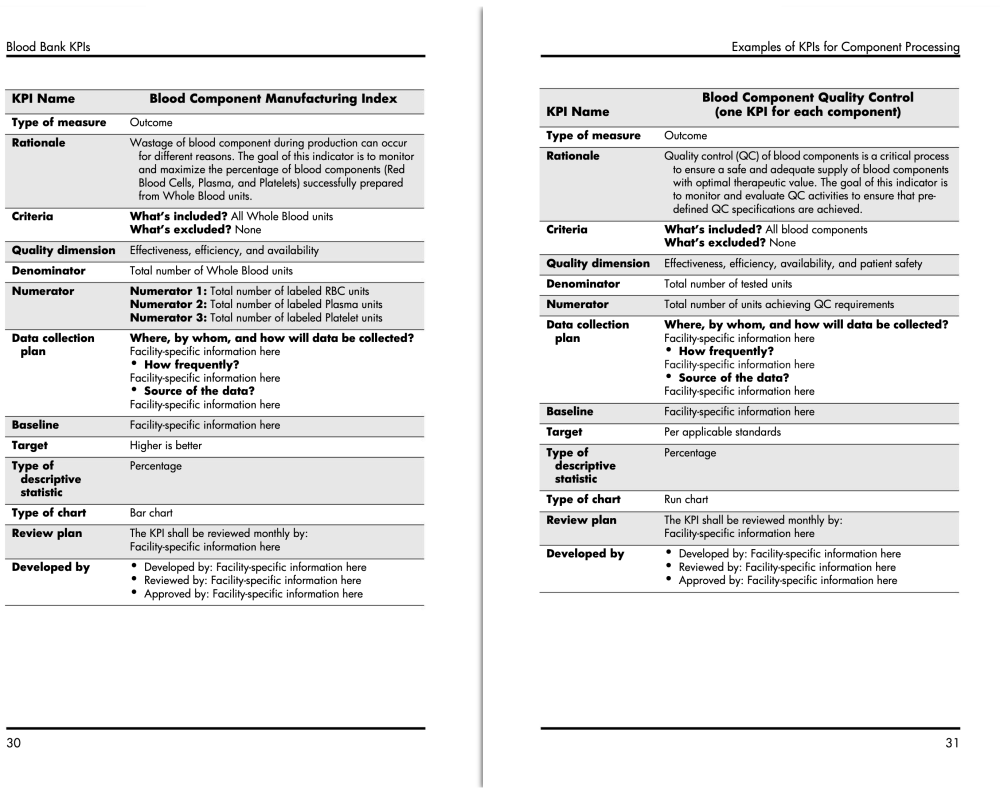
I don't currently work at a Donor Center, but I do have an AABB book for KPI examples.
Here are 2 KPIs on blood component manufacturing and QC:
There are a whole bunch of other KPI's in the book. Is there something in particular you are looking for.
sources:
https://www.aabb.org/regulatory-and-advocacy/regulatory-affairs
Alsaqri, Faisal Saud Blood Bank KPIs . https://ebooks.aabb.org/pdfreader/blood-bank-kpis
-
On 11/4/2022 at 1:34 PM, CARMEN DELGADO said:
Since the change of platelet compatibility. What are the new acceptable Groups for an AB + recepient?
Carmen
Since AB+ people are considered the "universal recipient" , we give them any type platelets, usually starting with the one with the closest out date.
- CARMEN DELGADO and Ensis01
-
 2
2
-
There is this one thing, I don't know if it is new regulation or it has been around for a while. I think CAP doesn't want any cardboard boxes in the core lab. This is due to card board boxes being porous and prone to biological contaminants. Blood bank is usually the only section that use cardboard boxes, the saline cubes. Thus the blood bank needs to be in its own seperate room. I guess contamination is a big no-no for the core lab, but not so much for the blood bank. I will look for this regulation and see if I can find it.
-
On 10/17/2022 at 5:44 AM, jayinsat said:
I had this very scenario about a year ago and it turned out mom had an anti-Dia. It was not on any of our in lot screening or panel cells. I did as I suggested and ran a select panel against mother's plasma using expired panel cells and identified the Dia. The eluate on the baby was eluted the Dia also.
Do you do QC on your expired panels when you use them as selected cells? I was just curious. Thank you
-
If bacterial contamination is suspected due to the signs/symptoms of the patient(high fever, vomitting, tachycardia, etc..) and/or discoloration of the blood product, an initial gram stain is performed and 4 plates are inoculated with the blood, Blood agar, chocalate, mackonkey, and an anaerobic plate. Then we go from there.
Not sure about the reasoning of incubating the broth at 4 degrees C. Is that to rule in/out a cold agglutinin?
-
My 2nd job is only per-diem, but I have been working almost everyday on my days off from my 1st job. We are so short of staff, the Lab Manager has to fill in for several evening shifts herself. That means, she does her day shift work and then continues on to evening shift and goes home at midnight almost everyday. They are so happy to see me on my days off from my 1st job, because they sorely need the help. The only upside to all this is I am getting so much overtime $$$. That's about it.
-
On 10/13/2022 at 4:43 AM, jayinsat said:
Nancy,
I echo everything you said, but I am experiencing the same things in a 400 bed hospital in downtown San Antonio. This is not sustainable and some sort of major intervention needs to happen very soon. After 37 years, I want out of this field.
So sorry to hear that. That is really tough. The last 2 years have been a nightmare to so many people in the lab. Perhaps talk to Greg Abbott, maybe he can offer some solutions. He is a very helpful person. Him and I went for a walk the other day, talking about his plans for gun control and power grid regulations {eyeroll emoji).
-
-
There has been quite a few H1B Visa lab techs that were hired from the Philippines in the last couple of years. Most of them are pretty good workers. We also have been hiring travellers. That's basically it. The last couple of years have been pretty rough. At least I have been getting so much over time. I was able to buy a bunch of toys like guitars and gadgets and go on vacations and stuff.
-
29 minutes ago, RRay said:
I have only used blood bands at my current institution and am evaluating getting rid of them. They cause a scary % of redraws (nurses cutting the wrong band, or rebanding when transferring between units, etc) and delay in patient care when patient ID isn't at question. And they're expensive.
You are right, for all the reasons you have indicated. I have always used blood bank bands until my current position. At first I was hesitant on not using them, but now I realize that nurses do get them wrong all the time. And this caused so much delay for patient care. Especially these days when a good chunk of nurses in hospitals are travelers, you need to train them each time.... Ugh, it's so time consuming.
-
20 minutes ago, RRay said:
Thanks for your feedback! I'm surveying the audience to see what can be pieced together as most efficient. Currently, my LIS requires override when a specimen being used for XM has not had a T&S on it. This LIS is going away next year tho.
I think that is a pretty standard and good safeguard for a LIS to have. What are you trying to XM? RBCs? Does your LIS do electronic XM? I assume you need additional sample because you need to do an AHG XM? Was the patient's current type and screen positive? Does the patient have a history of antibodies and what are they?
-
At my current place of work, there is a form/document we use to indicate that the specimens drawn are additional samples. These samples get their own unique specimen number and they are ordered as additional specimens. There are only valid if the patient's type and screen is current (within 3 days). My LIS support these documents because additional sample is an order with its own order code. Of course, all samples must have the patient's name, ID number, etc... to prove every sample are from the same patient.
I use to work for a blood bank that uses blood bank band numbers. I think most places in America uses blood bank band numbers. It is basically the same idea as above, except that they don't have "documents" to indicate the samples as additional samples. Instead, the additional specimens are labeled with the patient's blood bank band number and are only valid if the type and screen is current.
I hope this helps. Thank you
-
On 10/5/2022 at 6:36 AM, Malcolm Needs said:
Did you ever hear again from Mr. and Mrs. Lettuce Picker? Or know what became of baby Lettuce Picker?





Stumped by CDP
in Transfusion Services
Posted
I got the chloroquine method from the most recent AABB Technical manual. Maybe it can help you. Good luck.
METHOD 2-20. DISSOCIATING IgG BY CHLOROQUINE FOR ANTIGEN TESTING OF RED CELLS WITH A POSITIVE DAT RESULT
Principle
Red cells giving a positive direct antiglobulin test (DAT) result cannot be tested accurately with blood typing reagents that require an indirect antiglobulin technique. Under controlled conditions, chloroquine diphosphate dissociates IgG from the red cell membrane with little or no damage to its integrity. Use of this procedure permits complete phenotyping of red cells coated with warm-reactive autoantibody, including tests with reagents solely reactive by indirect antiglobulin techniques.
Specimen
Red cells with a positive DAT resulting from IgG coating.
Reagents
1. Chloroquine diphosphate solution prepared by dissolving 20 g of chloroquine diphosphate in 100 mL of saline. Adjust to pH 5.1 with 1 N NaOH, and store at 2 to 8 C.
2. Control red cells carrying a single-dose expression of antigens for which the test samples are to be phenotyped.
3. Anti-IgG antiglobulin reagent.
Procedure
Step
Action
1
To 0.2 mL of washed IgG-coated cells, add 0.8 mL of chloroquine diphosphate solution. Similarly treat the control sample.
2
Mix and incubate at room temperature for 30 minutes.
3
Remove a small aliquot (eg, 1 drop) of the treated test cells and wash them four times with saline.
4
Test the washed cells with anti-IgG.
5
If this treatment has rendered the cells nonreactive with anti-IgG, wash the total volume of treated test cells and control cells three times in saline and make a 2% to 5% suspension in saline to use in subsequent blood typing tests.
6
If the treated red cells are reactive with anti-IgG after 30 minutes of incubation with chloroquine diphosphate, Steps 3 and 4 should be repeated at 30-minute intervals (for a maximum incubation period of 2 hours), until the sample tested is nonreactive with anti-IgG. Then proceed as described in Step 5.
Notes
1. Chloroquine diphosphate does not dissociate complement proteins from the cell membrane. If red cells are coated with both IgG and C3, only anti-IgG should be used in tests performed after chloroquine treatment.
2. Incubation with chloroquine diphosphate should not extend beyond 2 hours. Prolonged incubation at room temperature or incubation at 37 C may cause hemolysis and loss of red cell antigens.
3. Some denaturation of Rh antigens may occur.
4. Many serologists test chloroquine-treated control cells for each antigen of interest. Select control cells that are positive for the antigen corresponding to the antisera that will be used to type the patient’s cells.
5. Chloroquine diphosphate may not completely remove antibody from sensitized red cells. DAT results on red cells from some persons, particularly those with a strongly positive initial test result, may only be diminished in strength.
6. In addition to its use for removal of autoantibodies, this method can be used for removal of Bg (HLA)-related antigens from red cells. Appropriate Bg controls should be used.
7. If a commercial kit is used, manufacturer’s instructions should be followed for testing and controls.