Content Type
Store
Profiles
Forums
Blogs
Events
Frequently Asked Questions
Gallery
Downloads
Glossary
Links Directory
Questions
Jobs
Vendors
Posts posted by SbbPerson
-
-
If they want RBCs without a confirmation, they will get uncrossmatched RBCs. I think an immediate spin crossmatch for an unconfirmed blood type is not useful.
Our same day surgery order extended type and screens if needed. They are good for 30 days out, just as long as the patient hasn't had a transfusion in the last 3 months. If the patient need a confirmation, a 2nd specimen will be collected prior to or on the day of surgery.
-
So that's the answer!? The front type was O Pos because the transfused cells were at the top. That's wild hehe. Great topic, very interesting.
-
I just answered this question.
-
My ScorePASS
-
-
Hello. When you say manual setup and read, do you mean with tubes?
-
marketlab.com has been a staple for lab supplies for as long as I can remember
-
WELCOME RDL

-
I just answered this question.
-
My ScorePASS
-
-
Just now, SbbPerson said:
I just answered this question.
-
My ScoreFAIL

-
-
I just answered this question.
-
My ScoreFAIL
-
-
This is a darn interesting question!

-
I just answered this question.
-
My ScorePASS
-
-
I attached a guide on how to set up a QC and Calibration program. Sartorius also have a calibration program where you can send them your E-pipet for them to calibrate it for you.
Here is the website address for that service:
https://www.sartorius.com/en/products/pipetting/pipetting-resources/pipettes-buyers-guide#id-1160668
Good luck.
pipetting-quality-control-application-guide-en-l-sartorius.pdf
-
Our gel cards are only IgG gel cards. We don't have the C3 gel cards. To QC our IgG gel cards, we use comb cells are a postive control and A1 or B cells as the negative control.
For the Poly(AHG), we use coomb cells and C3 cells for the positive control, and A1 or B cells for the negative control.
For Anti-IgG(AHG), we use coomb cells for the positive control, and A1 or B cells for the negative control.
I hope this helps. Good luck.
-
If the patient's antibody screen is negative, you won't be needing a segment from the blood on the helicopter. You can perhaps do an electronic crossmatch in those cases. We do the crossmatch retroactively when we receive the patient's sample.
But like what Jay said, if the screen is positive or the patient has a history of Ab's, you will need a sample of that transfused donor blood to do an AHG crossmatch, antigen typing, etc...
Do you know who will provide the blood for the helicopter? Our blood provider is also a transfusion servicer/IRL, so they saves samples of all units provided. They can send us a sample of the donor blood so we can do our crossmatch. In the case of the patient having an antibody/Ab history, we do the crossmatch retroactively after we get a patient's specimen and a sample of the donor's blood. Good luck.
-
I just answered this question.
-
My ScorePASS
-
-
1 hour ago, cthherbal said:
We contract the service to an outside vendor, but we review their compliance reports at transfusion committee meetings quarterly. Very low usage here (4 collections in 2022 with no blood returned to patients).
Did the 4 collections use a cell saver? If not, what process or instrument was used for collection? Thank you
-
Our cell saver machines are in the OR. Only surgery techs/personnel are allowed to use it. I never seen one in action but I hear good things. Attached are some guidelines, best practices, and indications for use. Good luck.
cell_saver1.pdf cell_saver2.pdf Guideline_IntraoperativeCellSalvage.pdf Poster_Abstract_Fatreduction.pdf US-Cell-Saver-Elite-Owner's-Manual-120859-AC.pdf
-
Welcome
-
46 minutes ago, AMcCord said:
Somewhat related question..... I need to replace the liners in my Credo coolers and order a couple of RT TICs. However I haven't been able to make contact with their sales people over the past year. No response to phone calls or emails. Can anyone give me a contact with someone that can help us?
Thanks!
Attached is the website and customer service number if you haven’t tried those. Good luck Credo Operating Room Container Product Sheet.pdf
-
We use the credo operating room containers. The container should be able to sustain 1 to 6 degrees celsius for 10 hours with the plates in them. We use this for surgeries when they need 1-6 units of blood or plasma , and it is not optimal for them to keep running back and forth to the blood bank.
1) We store the plates in a sub zero freezer.
2) We keep about 2 sets of the plates in a 1-6 degrees fridge. They are good in the fridge for about 40 hours. After the 40 hours, we put them back in the freezer.
3) They need to be in the freezer for about 12 hours before they can be used or be placed in the fridge.
4) You need to wait about half an hour or so before you can use them after you take them out of the freezer. Because obviously it will be too cold if you use it right away.
So yeah, to validate that it is working, measure the temperature inside the container for 10 hours.I attached the product sheet. Good luck.
-
Also this test should only be performed on Rh negative moms. And do not use any other Anti-D reagent, use only the one included in the kit.
Anyways, I attached the AABB procedure to this test. I hope this helps a little. Good luck.
-
On 12/28/2022 at 8:33 AM, paddleking said:
We count under slide. We wash with buffered saline, but not with PhiX added to unbuffered. We purchase isotonic phosphate buffered saline from thermo scientific. We have a sister hospital that follows the same procedure and reagents and they have not had issues. Talking to Immucor we are the only customer that has reported issues with this. We have gone through 3 lots. I have checked the pH of the saline and it is 7.02, which is perfect. We have washed both manually and with automated cell washers.
Anyone have any additional thoughts or directions I should go?
Does your sister hospital uses the same Lot numbers of Immucor FBS kit? If yes, then you guys must be doing something wrong. When was the last time your cell washer/centrifuge was calibrated? RPMs? Amount of saline dispensed?
If they are not using the same Lot #'s, perhaps give them one of your kits and see if they get the same result. If the kit's QC passes for them, then there is something wrong with either your procedure or maybe equipment. This is really strange. Good luck.
- Ensis01 and paddleking
-
 1
1
-
 1
1
-
On 12/16/2022 at 1:16 PM, CARMEN DELGADO said:
Yes. I was surprised that they were saying that we now needed two staff for verification. Thank you. Is there a standard or reg that speaks to that effect?
Carmen
I couldn't find a regulation that says that per se, but according to the AABB Standards for BBTS (33rd) edition;
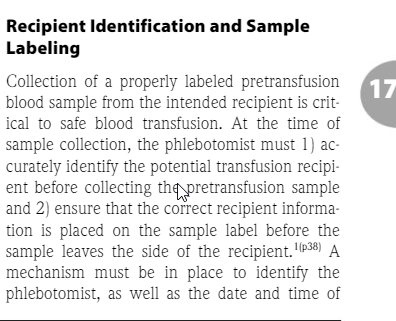
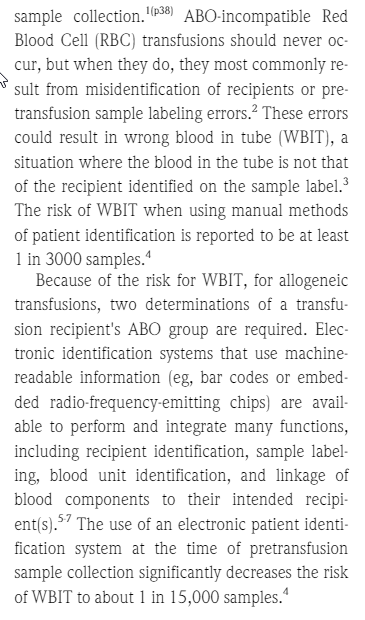
5.11.2.4 : The transfusion service shall have a policy to reduce the risk of misidentification of patient pretransfusion samples.
Also according to the recent AABB technical manual; page 504;
 In our facility, the phlebotomist and a 2nd person verifies the patient's ID at the time of the pretransfusion specimen is drawn. They both sign their names on the request for blood bank testing form.After the blood bank receives the specimen, me make sure the information matches between the form and the specimen. If the patient doesn't have ABO/Rh history, confirmatory ABO/Rh testing needs to be done before blood products can be issued.
In our facility, the phlebotomist and a 2nd person verifies the patient's ID at the time of the pretransfusion specimen is drawn. They both sign their names on the request for blood bank testing form.After the blood bank receives the specimen, me make sure the information matches between the form and the specimen. If the patient doesn't have ABO/Rh history, confirmatory ABO/Rh testing needs to be done before blood products can be issued.Sources:
Cohn, Claudia S., Delaney, Meghan, Johnson, Susan T. and Katz, Louis M.. <em>Technical Manual, 20th edition</em>. https://ebooks.aabb.org/pdfreader/technical-manual-20th-edition50155278
-
Strange , we have never had that problem. We use immucor too. I agree with AMcCord, washing is critical for this test. Also, how are you counting the agglutinates? By tube or slide? If you see agglutinates by tube, you should do it also on slide so you can count the number of agglutinates per LPF. If you get less than 5 agglutinates in 5 fields, it is negative.
- AMcCord and David Saikin
-
 2
2



Best Practice Alerts for Transfusion Reactions
in Transfusion Services
Posted
We just indicate in the test result , that the patient has history of of transfusion reaction. We are don't alert anyone because of course we are not physically present with the patient during transfusion. All our providers are very well versed on what to look out for during a transfusion, i.e. hives, chills, rash, fever, etc..