Content Type
Store
Profiles
Forums
Blogs
Events
Frequently Asked Questions
Gallery
Downloads
Glossary
Links Directory
Questions
Jobs
Vendors
Posts posted by SbbPerson
-
-
On 7/17/2023 at 7:44 AM, yan xia said:
I guess this may be caused by the Wharton's Jelly which can cause rouleaux formation of the cord cells. For the gel technique, it is good to test the adult cells without washing, but for the cord cells, maybe the Wharton's Jelly block antigens on the cells' surface. I noticed that patients suffer from Multiple myeloma will not show false positive reaction in gel, but saddly I have not confirmed if there are false negative reactons.
Yes, I was thinking wharton jelly too. This was a cord blood sample. Thank you
-
On 7/18/2023 at 9:17 AM, Sherif Abd El Monem said:
📚 Exciting News! Calling all blood collection and transfusion professionals! The highly anticipated 21st edition of the Technical Manual is now available. Stay on top of the latest developments, including updates on COVID-19 impact and molecular testing.
https://immunohematologymadeeasy.com/technical-manual-21st-edition/
Will you be selling this book? Thank you
-
I just answered this question.
-
My ScorePASS
-
-
On 5/28/2008 at 7:14 AM, csjuarez said:
I am in the process of evaluating performing cord blood testing on gel. We perform ABO/Rh type and DAT on babies born to type O and Rh Negative mothers. Our current testing is by tube and the DAT is evaluated microscopically. I was concerned that we might end up with more positive DATs in gel, but I'm finding the opposite! Almost all of the positive DATs with weak (microscopic) reactions are coming up negative in the gel (IgG).
If we convert to gel and "miss" these weak reactions, will that be significant? Do others read DATs microscopically? Are any of you doing Cord Blood testing in gel?
I know this post is about 15 years old, but I recently came across a similar issue. I have always done DATs in tubes. But currently switched to gel. I am use to washing the cells when I do a DAT. I find it kind of odd that the package insert for the IgG cards doesn't include washing in it's procedure. Do anyone know exactly why? The insert said just to straight add 10uL of packed cells to 1.0 mL of your MTS diluent to get your 0.8% cell suspension.
I got a weak positive reaction on a baby cord blood. I decided to wash the cells and the reaction came out stronger. Then I repeated this 2 more times and got similar results (see picture). All controls were negative.
Has anyone experienced this ? And can I get your thoughts on this matter? Thank you so much, please have a nice day.
-
On 6/28/2023 at 5:46 AM, beverleyj said:
Can anyone tell me what their informed consent process is for a blood transfusion in Ohio? Ohio law says that it must be obtained by the ordering provider. This is very hard to do in a hospital setting.
Not sure why is this very hard, maybe I am missing something. The patient is informed about the transfusion and all the risks involved and then they give or not give their consent. It is very hard in Ohio for the provider to get a consent form signed?
-
On 6/8/2023 at 10:47 AM, Ensis01 said:
I have never encountered a patient that says they have antibodies unless they have a card.
Several years ago, I met one patient with a card, She showed it to me when I drew her blood for a type and screen. I think she had Jka. She had no history and was visiting from out of town. Her physician recommended for her to keep her card on her person. First time I ever saw such a card.
-
I just answered this question.
-
My ScorePASS
-
-
On 7/15/2010 at 5:03 PM, gene20354 said:
Some time ago I asked for advice on validating Immucor's Anti-C3d with Ortho's bufferd gel card. I got the help that I needed but I have run into a problem. I have tested 4 samples that produced weakly positive to 1+ reactions with anti-C3d in the tube but all were negative when I tested them in gel. I have been converting Immucor’s complement control cells to 0.8% and using them as my positive control. The control always works but I can't figure out why my samples are coming up negative. Has anyone experienced this?
I know your post is 13 years old, but it got me thinking. Usually we QC out Anti-C3 reagent with the tube method, but I was curious to see what would happen if I used buffered gel card instead. This is what happened:
1) I converted my 3% C3 control cells to 0.8%.
2) I pipetted 50 uL this 0.8 cells into 2 wells of the buffered gel card, one labelled "pos" and the other "neg".
3) I pipetted 25 uL of Anti-C3 into the "pos" well.
4) I pipetted 25 uL of saline into the "neg" well.
5) I centrifuge the card for 10 minutes.
The results are as expected. The "pos" was 4+ and the "neg" was 0.
This was a stronger positive result compared to the tube method, in which we usually get is a 1+ to 3+ reaction.
My conclusion is that maybe there was something wrong with your gel cards. Did you use MTS diluent or saline when you converted your cells to 0.8? I am not sure if that would matter, but considering your samples were weakly positive to begin with, maybe the conversion "diluted" the samples.
-
I found the really good video on CLIA competency assessment. Maybe it can help a little bit
-
On 6/1/2023 at 2:42 PM, DPruden said:
Does anyone have a reference for manual isohemagglutinin titers? I can't find one on the AABB website or in the technical manual
For the manual isohemaglutinin(naturally occurring antibody) titration procedure, I attached the AABB method for it. Also the AABB method for early detection of hemolytic disease of the newburn. These procedures are from the current AABB methods manual.
Method_3_15_manual_titer_procedure.docx Method 5_3_Titer_for_HDN.docx
-
I just answered this question.
-
My ScoreFAIL
-
-
I just answered this question.
-
My ScorePASS
-
-
I just answered this question.
-
My ScorePASS
-
-
I just answered this question.
-
My ScorePASS
-
-
7 hours ago, John W. said:
I work as part of a 10 hospital health system. Currently, antibody identifications are sent to the main campus to perform usually as a costly STAT transport. We are looking at implementing antibody identification at the satellite hospitals but the cost of the additional anti-sera is pushing this to be more expensive than the STAT transport. All satellite sites send RBC unit segments to our main campus, who performs all the non-ABORH antigen testing for RBC units for the other 9 hospitals. Have you heard of having site A perform the antibody identification but then have the patient antigen test sent to site B?
I've been going through AABB Tech Manual/Standards and AABB/CAP Checklists and cannot find anything that explicitly states patient antigen typing must be performed, but should be used to support the antibody identification.
Scenario:
Patient ABID performed at site A (satellite). Site B (main) performs antigen typing for RBC units at site A. Site B notifies site A which unit to crossmatch. Site A crossmatches RBC unit, compatible. Patient antigen typing sent to Site B on routine courier to be performed within 24 hours.
Also, to reduce competency/proficiency testing needs, we are looking at having the satellite sites loading the ABID panel on their Echo analyzer but having the main campus still perform actual antibody identification/interpretation. Satellite site would print off the Echo result and fax/email the result and antigram to main campus. Has anyone else attempted this? If yes, what were your biggest hurdles? We are looking into ImmuLINK to allow the main campus to have access to the satellite Echos, but this probably will not make it through financial approvals
 .
.
Under the AABB Standards for Blood banks and Transfusion services(1st. ed. 04/2021), the recipients' red cells must phenotypically match the donor cells, i.e. it does not contain the corresponding antigen(s) to the recipient's antibody(ies).
I think the main idea is that the best blood to give to a patient is "type specific" blood. So that include the ABO/Rh antigens and all other clinically significant antigens that may trigger an immune response.
5.14.3When clinically significant red cell antibodies are detected or the recipient has a history of such antibodies, Whole Blood or Red Blood Cell components shall be prepared for transfusion that do not contain the corresponding antigen and/or are serologically crossmatch-compatible to include anti-globulin testing. -
15 hours ago, AMcCord said:
They probably barely have enough staff to cover what they cover now if they are a small enough facility to not have a night shift.
I am sorry, but I think that is a poor excuse. "I am sorry, your son died because we are under staffed." If a service is not available, do not offer it. Personally I think if a facility is not equipped to take care of a bleeding patient, they should not accept that patient in the first place. Especially for an ED that can't wait 20 minutes for blood. They do more harm than good. This just has lawsuit written all over it. I don't mean to offend anyone, this is just my opinion. Thank you
-
On 6/1/2018 at 6:45 AM, AuntiS said:
It looks like Sandra has the antibody answer key for each case. There are more questions than that, because it is over 500 pages. The book is "only" $80 on the website. But I bought it several years ago, so the price might have gone up by now
-
3 hours ago, Leyah said:
I actually need all the answers if you have time. 🙏
All of them? The book is over 500 pages, sorry, there are too many.
-
Why not just put a lab tech on night shift? That solves all your problems. You got to weigh your options and what do your prefer, a patient having life saving blood, or going over budget on salary/employee expenses. Good luck
-
I have it, I bought it years ago from the AABB website. I may have to search for it a little. Do you have a particular question or section in mind? There are a lot of questions.
-
Yes, it is. It is actually because the transport temperature for whole blood, RBCs, and plasma is 1 to 10 degrees celsius. After 30 minutes, the internal temperature of the units go above 10 degrees which may promote bacterial growth. Basic 30 minute rule is that if the unit is outside of a controlled temperature for 30 minutes or more, it must be discarded.
-
Do you have an on-call lab person? Most hospitals with an emergency room have a lab person on-call when the lab is closed. They can come in and issue the blood. If not, call the lab manager/supervisor and make them come in and issue you the blood.
There is the HaemoBank that is fairly easy to use and perhaps can be used to store just uncrossmatched emergency blood. Maybe you can get one of those. I know some smaller hospitals that use it. It's like a small refrigerator/vending machine for blood products. Good luck
-
There are hospitals now that has switched their verbiage from "least incompatible" to "most compatible". Which is true! This blood is the BEST blood possible considering the patient's situation. There is nothing you can do about the patient's auto, but you can make sure to provide the "best" compatible unit to the patient. Of course you do this by making sure there are no underlying clinically significant alloantibodies in the patient's plasma.
Some places just straight out say "incompatible" on the transfusion report/tag. The physician is then notified and made aware of this. Some places make the doctor sign a form acknowledging the "incompatible" units and the risks involved, but where I work, a verbal "ok" would suffice. We are all on the same team, working towards the same goal, the welfare of the patient. We are not trying to "pin the blame" on anyone for possible hemolytic transfusion reactions. We all want the same thing.
Here is a really good podcast on the subject from the Blood Bank guy. It is really interesting and goes deeper into the subject and "what to do when everything is incompatible". Good day.
https://www.bbguy.org/2020/06/17/085/
- Mabel Adams and tms8313
-
 2
2
-
At my hospital we don't use bands. I think this is great, because our rejection rate is pretty low. There are too many travelling nurses we need to train if we were to use the bands. And even when trained, nurses still get them wrong.
Now all they need to do if to make sure the specimen has at least 2 unque identifiers , along with collectors's info, date, and time. Simple. Also for each type and screen, a testing request form is filled out. All patient information must match between the form and the specimen. That's it. Nice and simple.
The band is great, but not everyone knows how to use it, and training takes up alot of time, especially with all the travelling nurses we have.


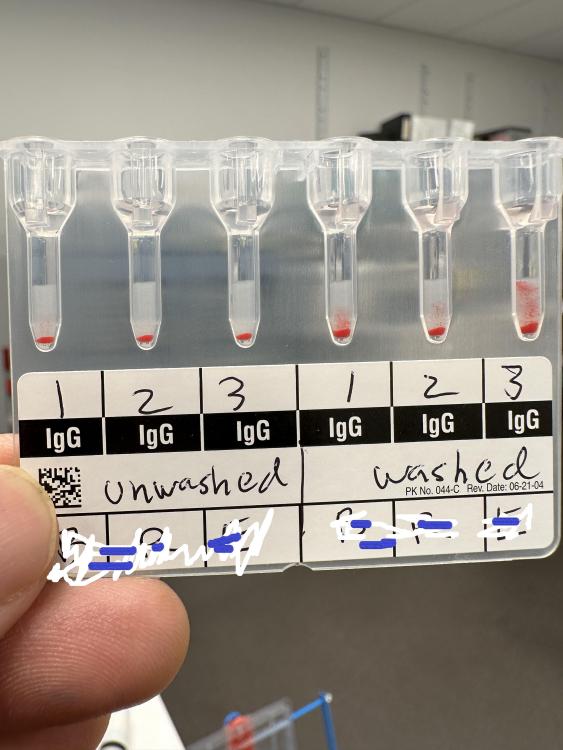
Cord Blood testing on gel
in Transfusion Services
Posted
I doubt it. We QC our saline in duplicate every day. Thank you for your input.