Content Type
Store
Profiles
Forums
Blogs
Events
Frequently Asked Questions
Gallery
Downloads
Glossary
Links Directory
Questions
Jobs
Vendors
Everything posted by BloodbankZ
-
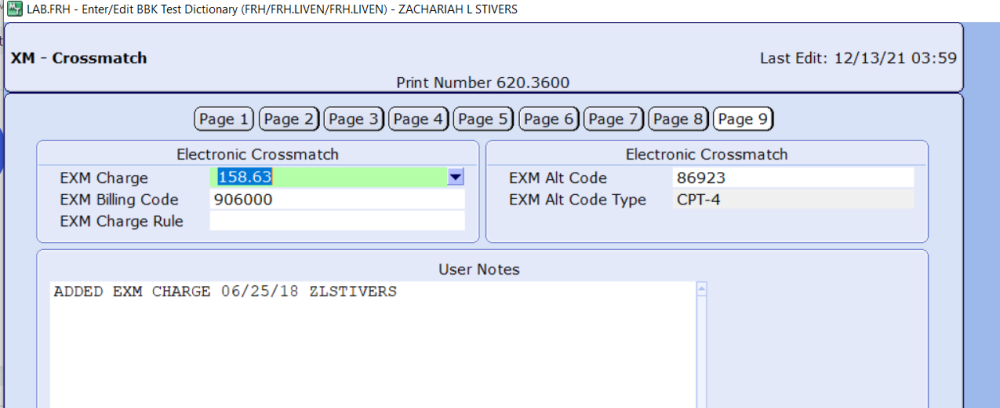
Electronic crossmatch with Meditech
BloodbankZ replied to BloodbankZ's topic in Computer Systems / Software / ISBT128
You will need to go to the BBK History desktop and then to Replace XM Test. Under it you will need to enter the history record number and put a Y in the Exclude from Electronic Crossmatch. Their are of course steps in LIS and BBK parameters but the billing is on this page below. -
I am looking into the billing for the CCP. We used 5 units under the EIND and I was able to find the attached file online. Has anyone else heard is there a HCPCS code yet? We are now in the Expanded Access Program and I know we will not be charged for those units. Does anyone know can you still charge for the thawing HCPCS#86927? TIA. Request-to-HHS-CCP-Payment-Pathway.pdf
-
Carrie Easley thank you. How did you seal the wire at the port entrance?
- 13 replies
-
- cooler
- thermometer
-
(and 2 more)
Tagged with:
-
I have tried a data logger the Microlite III temp logger. It did not seem to instantaneously read the cooler temperature but just the temperature that the logger itself had been at. Conditioning it at room temperature it took several minutes before lowering in temperature when placed in a cooler with ice. I pre conditioned it in the refrigerator and it took a long time to read a higher temperature at room temperature. We store blood products in a cooler at bedside during surgery and need to account for the temperature. Do you have any brand suggestions?
- 13 replies
-
- cooler
- thermometer
-
(and 2 more)
Tagged with:
-
I need to be able to monitor blood products while in a cooler such as at bedside in the OR during a surgical case. We currently use the BT10 indicators but I am needing a device to take a digital temperature reading of the cooler at intervals during the surgery. Does anyone have any suggestions? Thanks in Advance!
- 13 replies
-
- cooler
- thermometer
-
(and 2 more)
Tagged with:
-
Remote as in another location within the same hospital outside of the Blood Bank like the ER or OR.
- 4 replies
-
- trauma blood
- blood in er
-
(and 2 more)
Tagged with:
-
I am looking at storing blood products in a remote location for our facility. Just curious what everyone is doing currently? Thanks for the info!
- 4 replies
-
- trauma blood
- blood in er
-
(and 2 more)
Tagged with:
-
We use the Ortho Confidence System and AB plasma as our negative control. That will cover all of your possibility of reactions. Yes you would have to QC the ABD card as well. For Cord study we run the ABD w/Rev (in case the patient is AB POS you need the ctrl) and an IgG card well for the DAT. It may detect weak D but we perform a weak D test on every Rh negative newborn.
-
We use Ortho gel primarily. It states nothing about centrifuge time. We use greiner tubes and they recommend 2000g for 10 min for platelet poor plasma. I am leaning towards the Stat spin express which is 3 minutes at 4000g. As long as it validates I don't see a problem do you?
- 9 replies
-
- stat
- centrifuge
-
(and 1 more)
Tagged with:
-
Is there still a good serological centrifuge out there?
BloodbankZ replied to Mabel Adams's topic in Transfusion Services
Check EBAY I picked up a Clay Adams Serofuge there to "revitalize" one of ours. I think it was only $400. -
BankerGirl do you know the g force?
- 9 replies
-
- stat
- centrifuge
-
(and 1 more)
Tagged with:
-
I am looking to cut down our TAT. We currently spin our specimens for 10 minutes. Any suggestions for stat centrifuges? TIA.
- 9 replies
-
- stat
- centrifuge
-
(and 1 more)
Tagged with:
-
I am needing some help setting up electronic crossmatch in Meditech. I have everything else in place but can not figure out how to prevent EXM in patients who have mixed field reactions. I am wanting to be able to perform the IS serologically as I understand this is needed per CAP and FDA regulations. Has anyone else experience this. We are currently on version 6.08. Thanks in advance!
-
DTT-Treatment of Screening Cells (Daratumumab/Darzalex)
BloodbankZ replied to septima's topic in Transfusion Services
I would say it is due to the DARA. DTT treated cells will eliminate the DARA reactivity and let you be able to rule out everything besides mainly Kell system which DTT denatures. We perform a baseline type and screen and DNA HEA typings on patients before they start. If DTT screen is negative we give K negative cells if patient is antigen negative.- 36 replies
-
- daratumumab
- dara
- (and 4 more)
-
DTT-Treatment of Screening Cells (Daratumumab/Darzalex)
BloodbankZ replied to septima's topic in Transfusion Services
When I tried to run it in gel I never could get nice clean reactions. Their would always be particulate at the top of the wells. I have seen several tube procedures that are just using so many drops of 4% cell suspensions instead of concentrating their cells and then getting an exact 4:1 ratio with DTT. I have since went to doing it in tube. What kind of steps are you performing?- 36 replies
-
- daratumumab
- dara
- (and 4 more)
-
Thanks so much Eman that is just what I needed. I just couldn't find it.
-
I was wondering if anyone knew of a report that showed incident rate of FDA reportable events per hospital or per other statistic like transfusions, patients, etc... Just looking for a baseline to see how our facility compares to others. I have searched and reached out to other facilities and haven't had any luck. Thanks in advance.
-
DTT-Treatment of Screening Cells (Daratumumab/Darzalex)
BloodbankZ replied to septima's topic in Transfusion Services
Has anybody tried DTT treating reagent red blood cells in MTS gel method? If so are you concentrating your cells before treating or have adjusted your ratio of DTT to the .8% suspension? TIA.- 36 replies
-
- daratumumab
- dara
- (and 4 more)
-
What are other people's institutions practices on the following. If you have a patient with an anti-D do you need to go ahead and carry out the D antigen typing on the patients rbcs through the IAT phase(weak D testing)? The AABB 18TH ed. Technical Manual states on pg. 327 "When the D type of a patient is determined, a weak D test is not necessary except to assess the red cells of an infant whose mother is at risk of D immunization." It then goes on to say under Identification of Antibodies to Red Cell Antigens pg.401 "Determining the phenotype of the autologous red cells is an important part of antibody identification." We use MTS gel for as our primary method for blood type determination and it states that Most weak D antigen expressions will be detected(which means not all), however partial DVI epitope variant of the D antigen will not be detected with this monoclonal reagent. Not that it really changes how we transfuse the patient but just curious to others procedures/thoughts. Thanks in advance.
-
We have been using Ortho Gel since 2008 we have serviced our Tipmaster pipettes several times. We are needing something new has anyone used the Vista Ovations for Ortho Gel or have another suggestion. Thanks.
-
Okay so I was always taught to use the rule of 3, 3 positive reactions and 3 negative reactions for peforming an antibody ID. I was also taught to always use homozygous positive and negative cells whenever possible. Sometimes of course it is not due to low incident/high incident antigens. I do know you need to use a homozygous cell when performing "rule outs". What is everyone else's practices and thoughts as I need to clarify our current antibody identification policy. Thanks in advance.
- 7 replies
-
- homozygous
- heterozygous
-
(and 5 more)
Tagged with:
-
Issuing multiple units to one patient
BloodbankZ replied to BloodbankZ's topic in Transfusion Services
To clarify if we do issue more than one unit they are placed in a cooler with appropriate temp control. Also our coolers are validated for 24 hrs. -
Hi I am creating a policy on issuing multiple units to one patient. We already do this for the ER, OR, CVICU, and ICU, and on any emergent need. But now we are getting requests for just general practice. Does anyone know of any AABB, FDA, or CAP standard about this I am having trouble finding one as far as who should and shouldn't get it. I am not in support of doing it just for non patient convenience thanks in advance.